

Polymerized silicate melts behave anomalously at high pressures, in
that their viscosities decrease with increasing pressure. While viscosity
measurements at high pressures are experimentally challenging (most studies
to date report viscosities only up to ~ 3 GPa),
it has been shown that viscosities can be estimated from the diffusivities
of network forming ions using the Eyring relation. We have determined oxygen
self diffusion coefficients up to 15 GPa for three melts with varying degrees
of polymerization. We find that for sodium tetrasilicate (Na2Si4O9)
which nominally has an average of 0.5 non-bridging oxygens per tetrahedral
cation (NBO/T), oxygen diffusivities continue to increase with pressure
up to 15 GPa. Results from molecular dynamics simulations of Na2Si4O9
liquid have indicated that diffusivities pass through a maximum near 20
GPa. For the slightly more polymerized Na3AlSi7O17
(NBO/T = 0.25), the experimentally determined diffusivities pass through
a maximum near 8 GPa. For albite melt (NaAlSi3O8)
for which NBO/T = 0, we observe a diffusivity maximum near 5 GPa (Figure
3.8-1). These results thus suggest that viscosities pass through a minimum
with increasing pressure, and that the minimum is polymerization dependent.
Solid state NMR studies have shown that glasses quenched from high pressures
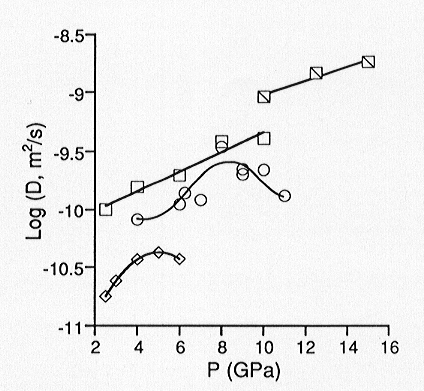 |
Fig. 3.8-1: Pressure dependence of oxygen diffusivity in silicate melts of composition NaAlSi3O8 (diamonds), Na3AlSi7O17 (circles), and Na2Si4O9 (squares) at 1800 °C. Hatched squares represent diffusivities for Na2Si4O9 at 2200 °C from 10 to 15 GPa. |
contain increasing amounts of high-coordinate Al (in aluminosilicates) and Si (in binary silicates) with increasing pressure. We interpret this as a densification mechanism which initially enhances oxygen diffusivities, as non-bridging oxygens are likely to participate in the formation of high coordinate Al and Si species. However, at higher pressures, after all non-bridging oxygens have been consumed by this reaction, bridging oxygens are required to continue increasing the coordination of Al and Si, thus forming three-coordinate oxygen. This marks the diffusivity maximum (and viscosity minimum) and accounts for its polymerization dependence. Such that a diffusivity maximum is observed at all for the fully polymerized albite melt could indicate that oxygen self diffusion is sensitive to trace amounts of water. FTIR spectra of the quenched products reveal the presence of water. However, there is little variation in water content as a function of pressure, and the estimated concentrations (<0.1 wt%) would reflect only a minor extent of depolymerization by incorporation of H2O. Finally, increasing oxygen diffusivity with pressure up to 5 GPa is consistent with decreasing viscosity up to 3 GPa which has already been observed for dry albite melt.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page