

The solution behaviour of high field strength cations (e.g. Ti4+, Zr4+, P5+) in silicate melts is known to be very composition dependent. Spectroscopic studies have shown that the alkali/aluminium ratio is one of the major controls on the solution behaviour of these elements. Macroscopic property measurements (e.g. density, phase relations, viscosity) may also be used to provide constraints on the structural role of such cations in silicate melts. Viscosity is potentially very useful because of its sensitivity to melt composition. Although viscosity is in general controlled by entropic as well as enthalpic effects, for viscosities in the range 1-3 Pa s it has been shown that the configurational states available become so numerous that enthalpic effects become the dominant factor limiting viscous flow. Therefore, variations of viscosity caused by the addition of high field strength cations may be related to changes in the average bond strength of the melt, implying that different solution mechanisms of these cations should have different effects on melt viscosity. At present, the shear viscosities of 40 compositions in the system Na2O-Al2O3-SiO2-P2O5, and 24 in the system Na2O-Al2O3-SiO2-TiO2 have been determined in the temperature range from 1000 to 1650 °C using the concentric cylinder method. All the studied compositions lie along the 67 mol% SiO2 isopleth of the HFSE free system, and vary from peralkaline (Na/(Na+Al) = 0.7 to 0.54) through metaluminous (Na/(Na+ Al) = 0.5) to peraluminous (Na/(Na+ Al) = 0.47 to 0.44).
The effect of the addition of phosphorus on viscosity at fixed temperature
is very sensitive to the alkali/aluminium ratio, as shown in Fig. 3.8-4.
Addition of phosphorus to peralkaline melts results in an increase in viscosity.
With progressive additions of phosphorus to mildly peralkaline melts (Na/(Na+Al)
< 0.60), there is a maximum in melt viscosity which occurs at lower
phosphorus content as the peralkalinity of the melt decreases. In contrast,
the addition of phosphorus to the metaluminous and peraluminous melts causes
a decrease in melt viscosity. The magnitude of this decrease is identical
for the metaluminous and mildly peraluminous (Na/(Na+Al) = 0.47) compositions,
but smaller for the most peraluminous melt (Na/(Na+Al) = 0.44). Two main
conclusions concerning the interaction of phosphorus and other cations
are inferred from the viscosity data: 1) The addition of phosphorus to
peralkaline melts results in the formation of sodium phosphate complexes
which, upon exhaustion of excess sodium, have the stoichiometry of extended
metaphosphate chains with Na/P ratios which tend to 1 as the metaluminous
join is approached. 2) In the most peraluminous melts studied, phosphorus
is inferred to interact with both excess aluminium and network forming
aluminates, suggesting that these two species have similar energetic stabilities.
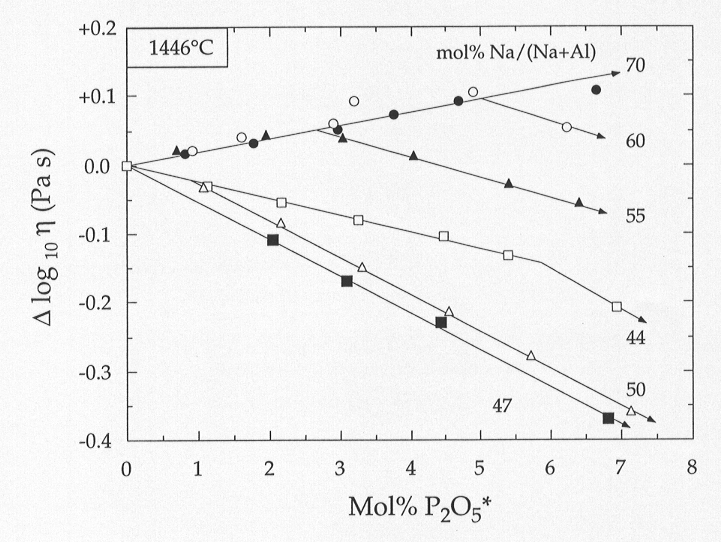 |
Fig. 3.8-4: The change in viscosity, Δ log10 ν, at 1446 °C as a function of added P2O5 to melts of variable sodium to aluminium ratio along the 67 mol% SiO2 isopleth in the system Na2O-Al2O3-SiO2. |
The results for the titanium bearing melts contrast with those obtained for phosphorus. At a fixed temperature the addition of titanium reduces the viscosity of all the studied melts. The viscosity decrease is a linear function of TiO2 for each of the four base compositions studied (for additions up to 15 mol% TiO2). However, the magnitude of this decrease is not constant, nor is it a linear function of the Na/(Na+Al) ratio of the melts, being greater for melts close to the metaluminous join. The number of moles of added titanium is much greater than the number of moles of excess sodium in the Ti-free base compositions. The linear decrease of viscosity over a large range of TiO2 contents is therefore difficult to reconcile with the proposal that formation of sodium titanate complexes is the only solution mechanism of titanium in these melts because, for all reasonable stoichiometries of Na-Ti complexes, evidence for a change in solution mechanism of titanium (e.g. a break in slope of viscosity as a function of TiO2 at fixed temperature as was the case for phosphorus) may be expected, but is not observed.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page