

A silicate melt in equilibrium with a hydrous fluid dissolves some water; the hydrous fluid, on the other hand, dissolves some silicate component. As these solubilities may increase with pressure and temperature, it has been suggested that at some point the composition of the two phases could become equal. Beyond this critical point, there would be complete miscibility between silicate melt and fluid. Such supercritical behaviour is known to occur in the SiO2-H2O system. However, it has been debated for a long time whether such a phenomenon is also possible in the geologically more important aluminosilicate systems. Here we report the first direct observation of supercritical behaviour in the albite (NaAlSi3O8) - H2O system.
For our experiments, we used an externally-heated diamond cell. At the
beginning of an experiment, we loaded a chip of hydrous albite glass and
a droplet of water into the cell. From the homogenization temperature of
the hydrous fluid upon heating, we determined its bulk density. This allowed
us to estimate the pressure inside the cell at any temperature using the
equation of state of water. Figure 3.5-1 shows four pictures of the system
albite-H2O in the diamond cell at 14.5 kbars and 763 to 766°C.
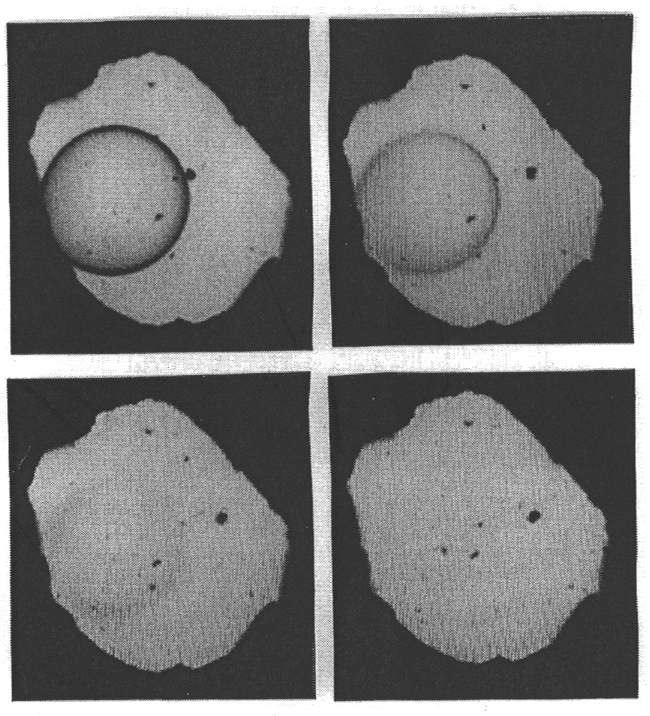 |
Fig. 3.5-1: The system albite-H2O in a diamond cell at 14.5 kbars and 763 to 766°C. At 763°C, a droplet of hydrous albite melt is surrounded by aqueous fluid (top left). With increasing temperature, the compositions of the two phases converge and the optical contrast between them diminishes (top right and lower left). At 766°C, the phase boundary has disappeared (lower right). |
Initially, a drop of hydrous albite melt is surrounded by hydrous fluid.
A slight increase in temperature makes the compositions of the two phases
converge which causes a reduction of the optical contrast between the phases,
until the boundary disappears entirely. By measuring infrared spectra through
the two phases inside the diamond cell, we were able to determine the water
contents of the coexisting phases as a function of temperature. The resulting
phase diagram is shown in Fig. 3.5-2. Finally, by varying the bulk density
of the sample in the diamond cell, we determined the dependence of the
critical temperature on pressure, i.e. the critical curve (Fig. 3.5-3).
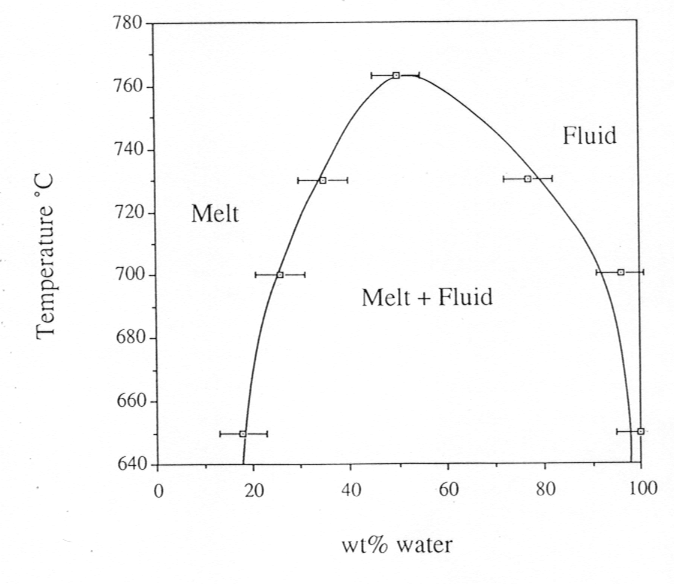 |
Fig. 3.5-2: Phase diagram for the albite-H2O-system under isochoric conditions. Pressure is 12.2 kbars at 650°C and 14.5 kbars at 766°C, the critical temperature. |
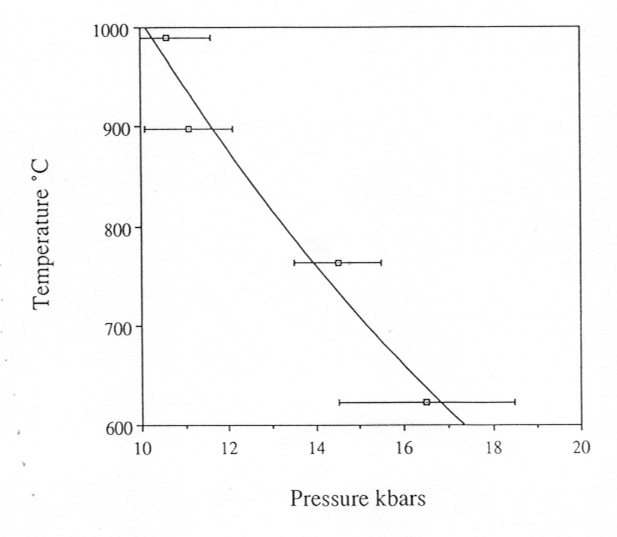 |
Fig. 3.5-3: The critical curve in the system albite-H2O. |
The composition of albite is very similar in SiO2 and Al2O3 content to hydrous partial melts of a wet peridotite at upper mantle pressures. It is therefore very likely that supercritical behaviour of silicate melt - hydrous fluid systems occurs at least in the deeper part of the upper mantle. This has fundamental consequences for the phase relationships in a hydrous mantle. At the solidus temperature of a water-saturated mantle, solid minerals coexist with a hydrous fluid and a silicate melt. Beyond the critical curve, however, melt and fluid cannot coexist as two separate phases anymore. This means that with increasing temperature the hydrous fluid coexisting with mantle minerals will dissolve more and more silicate component, until it reaches the composition of a hydrous silicate melt. There will be no discrete melting event and a water-saturated solidus temperature of the mantle cannot be defined anymore.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page