

In previous experiments on water solubility in pyroxenes we showed that the OH substitution is not necessarily coupled to chemical defects. Water solubility in pure MgSiO3 enstatite reaches 1000 ppm by weight at 1100°C and 100 kbar. In nature, numerous solid solution series and trace element substitutions are possible that could influence the incorporation of OH groups in the pyroxene structure. In order to understand the effect of chemical impurities, two series of experiments were carried out. The first set of experiments involves growing enstatite crystals at 1100°C and 15 kbars and water-saturated conditions from gels of enstatite composition doped with Al, Li and B. The second series consists of similar experiments with gels of diopside composition doped with Al, Li or B as starting material. Water contents in enstatite and diopside were measured with an FTIR-spectrometer. Unpolarised spectra were taken on clear, inclusion-free single crystals several 10 to 100 µm in size.
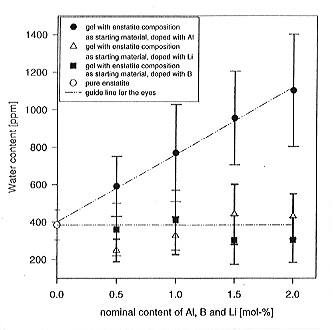
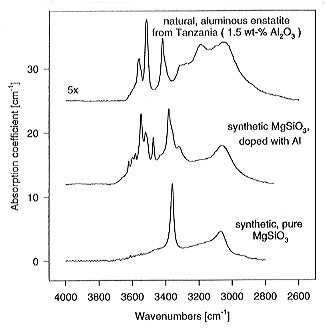
Figure 3.5-4 compares the water content of pure MgSiO3 enstatite and enstatite grown from gels doped with Al, B or Li. While boron and lithium have no effect, water solubility greatly increases with aluminum. A nominal substitution of 2 mol% Al for Si increases the water content from 380 ppm to 1100 ppm by weight, probably due to the substitution Al3+ + H+ for Si4+ on the tetrahedral site. In the near infrared spectra one can clearly distinguish OH bands arising from the intrinsic solubility from bands due to the coupled substitution of OH and Al. This in turn allows the OH substitution mechanism in natural samples to be identified (Fig. 3.5-5).
In diopside, water solubility increases only slightly with aluminum
content. However, all band positions shift to lower frequencies. This suggests
that the substitution Al + Al for Mg + Si is predominant, which changes
the lattice constants and interatomic distances and, in turn, the position
of the OH bands.
 |
|
 |
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page