

Shen and Keppler recently demonstrated that there is complete miscibility between hydrous fluid and silicate melt in the system NaAlSi3O8-H2O at high pressure and temperature, with the critical curve going through the points 989°C - 10.6 kbar and 623°C - 16.5 kbar. Beyond this critical curve, melt and fluid cannot coexist as two separate phases. The system albite-water is a simple model that is not directly applicable to phase relations in the upper mantle, because magmas generated by partial melting in the mantle are usually silica-undersaturated and contain magnesium. If the results obtained for albite-H2O were applicable to natural magma compositions, they would have fundamental consequences for phase relationships and mass transport in the mantle and possibly also in parts of the lower crust.
We carried out similar experiments with a wide variety of iron-free silicate melt compositions close to those found in nature. We synthesised nepheline (Na3KSi4Al4O16), jadeite (NaAlSi2O6), haplogranite (Qz0.35Ab0.4Or0.25), rhyolite (Qz0.32Ab0.36Or0.22An0.1), andesite and tholeiite glasses, containing about 5 wt% of water. Experiments were carried out in a modified Bassett-type externally-heated diamond anvil cell. For each experiment, the cell was filled with samples of glass (approximately half of the volume of the sample chamber), distilled water and an air bubble. Water served as the pressure medium, and the pressure in the cell was calculated using its equation of state.
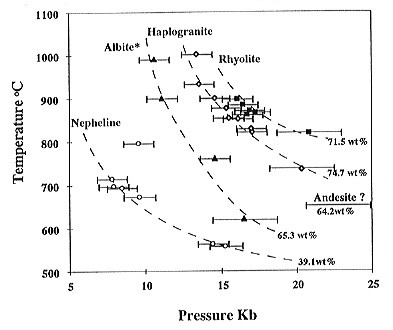
The same kind of critical behaviour as described by Shen and Keppler
for the albite-H2O system was observed for nepheline-H2O,
haplogranite-H2O, and rhyolite-H2O. The critical
curves go through the following points: haplogranite: 735°C, 20.3 kbar
- 1003°C, 13.4 kbar; rhyolite : 820°C, 20.8 kbar - 898°C, 16.1
kbar; and nepheline: 557°C, 15.2 kbar - 698°C, 7.8 kbar (Fig.
3.5-6). In all systems investigated, the critical temperature decreases
with pressure. At constant pressure, the critical temperature increases
with the silica content of the system. Experiments with andesitic and basaltic
compositions are complicated by crystallization during heating. Crystallization
also occurs during experiments in the nepheline-H2O system.
In this case, we dissolved the crystals at high temperatures (900°C)
and then decreased the temperature until fluid and melt phase separated.
The critical temperature can then be accurately measured by heating the
charge slowly until the phase boundary disappears. In the case of more
silica-undersaturated compositions such as andesite and tholeiite, the
temperature necessary to dissolve the crystals is sometimes not accessible
with the diamond anvil cell. This restricts our study to high pressures
(P > 20 kbars) because of the lower critical temperatures under these conditions.
Evidence for supercritical behaviour was observed in the system andesite-H2O,
with a critical temperature of 665°C. However, the bulk density of
this experiment was too high to be accurately measured; the pressure was
probably above 20 kbars. This would place the critical curve of andesite-water
close to that of albite-water, consistent with the similar silica contents
in these systems (Fig. 3.5-6).
 |
|
Our results clearly show that a wide range of silicate melts are completely miscible with water at the conditions corresponding to the deeper part of the upper mantle. This means that for example in the deeper part of subduction zones a continuous spectrum of fluid compositions ranging from silica-poor aqueous fluids up to hydrous silicate melts may be present, depending on temperature and pressure.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page