

Transition metals in silicate melts play an important role in the early stages of magma crystallisation, mineral fractionation and emplacement of ore bodies because of their influence on the rheologic behaviour of such melts and supercooled liquids. Here, particular interest is focused on the variation in glass transition temperatures (Tg), i. e. the plastic/elastic boundary, as a function of transition element enrichment. This is the critical control on rheological properties. For this study, dilatometric and calorimetric measurements have been carried out on Na-disilicate and anorthite-diopside (1 atm eutectic composition) glasses, containing up to 15 mol% NiO and up to 35 mol% CoO. Na-disilicate was chosen due to its rather simple glass structure, whereas anorthite-diopside represents a more realistic model for basaltic melts. The dataset, when complete, will be evaluated to yield molar expansivities (dV/dT) of the supercooled liquids up to 800°C, and thus help establish an equation of state for such liquids.
Preliminary results reveal that the glass transition temperatures of
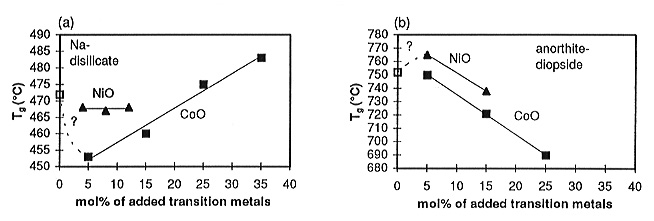
the Na-disilicate glasses (Fig. 3.6-7a) remain constant, within error,
with increasing NiO content. For CoO there seems to be a minimum in Tg
near 5 mol% and then the glass transition temperatures increase again with
CoO content. At the highest CoO content studied (35 mol%), Tg
is 12°C higher than for Na-disilicate. The anorthite-diopside glass
transition temperatures (Fig. 3.6-7b) decrease by ca. 50°C with increasing
CoO concentrations. The addition of 5 mol% NiO slightly increases Tg
of a anorthite-diopside melt, whereas adding 15 mol% lowers Tg
by ca. 15°C (Fig. 3.6-7). These trends may be explained in terms of
the structural effects of the added transition metal cations: in the anorthite-diopside
glasses, Co2+ acts as a network-modifier and increasing CoO
contents allow an anorthite-diopside melt to supercool and exhibit a liquid-like
behaviour at considerably lower temperatures than the undoped material.
The maximum in Tg for NiO added to anorthite-diopside glasses
could indicate a change in coordination from network-forming to network-modifying
behaviour. An additional complication, however, is the existence of Ni2+
in five-fold coordination (cf. Galoisy and Calas 1993, Geochim. Cosmochim.
Acta, 57: 3627-3633). Adding Co2+ to Na-disilicate melts seems
to suggest a network-modifying role at low concentrations but at higher
concentrations a network-forming character, indicating a coordination change
of the Co2+ cation. Apperently no effect within error of NiO
content on Tg is observed in Na-disilicate melts, which might
be due a range in coordination numbers of Ni2+ compensating
their effects on Tg.
 |
|
Volume expansions of the samples were measured in a quartz push-rod dilatometer during heating of the samples following an initial heating and controlled cooling. Thermal expansivities (dV/dT) of the glasses can be regarded as true values up to the glass transition temperature, which in this study is defined as the peak temperature of the volume expansivity. Due to the viscous deformation of the supercooled liquids above the glass transition, the expansion of the glasses cannot be measured directly in the dilatometric run.
The equivalence of volume and enthalpy relaxation permits the calculation of molar expansivities of the supercooled liquids from specific heat capacity (cp) data of the samples. To obtain the heat capacities, the samples were reheated after first cooling in a scanning calorimeter at cooling rates similar to those previously applied in the dilatometric run. By normalizing both the cp and dV/dT data to the peak values we can then calculate the molar expansivities of the supercooled liquids.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page