

Silicate perovskite probably constitutes more than half of the Earth by volume, since the lower mantle is believed to consist predominantly of this phase. Physical and chemical properties of silicate perovskite therefore largely determine the properties of the bulk mantle. The dominant component in the lower mantle perovskite phase is MgSiO3, but a number of additional cations substitute into the structure, including Fe2+, Fe3+, Al3+, Cr3+, Ti4+ and Ni2+. The nature of these cation substitutions will affect the physical and chemical properties of the lower mantle perovskite phase.
Three substitution mechanisms that produce perovskite-type structures can be considered: (A) self-compensated substitutions that do not change the oxygen concentration; (B) oxygen-deficient compositions formed when a cation substitutes for one with higher valence; and (C) oxygen-excess compositions formed when a cation substitutes for one with lower valence. Examples of these mechanisms for Fe3+ substitution into MgSiO3 are given here in Kröger-Vink notation:
| Fe2O3 + MgMg + SiSi -> FeSi' + FeMg. + MgO + SiO2 | (A) |
| Fe2O3 + 2SiSi + OO -> 2FeSi' + VO.. + 2SiO2 | (B) |
| Fe2O3 + 2MgMg -> 2FeMg + 2MgO + 1/2 O2 + 2e' | (C) |
where the electrons in mechanism C could be compensated for in several ways including cation vacancies or excess oxygen in the structure.
We have investigated the substitution of Fe2+, Fe3+ and Al3+ in MgSiO3 perovskite on samples synthesised under varying conditions of oxygen fugacity in the multianvil press at 26 GPa and 1650-1750°C for 2-8 h. (Mg,Fe)(Si,Al)O3 perovskite samples were characterised using X-ray diffraction, electron microprobe, and analytical transmission electron microscopy (TEM). Fe3+/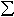 Fe values determined using Mössbauer spectroscopy and electron energy-loss spectroscopy (EELS) are in good agreement. Fe3+/
Fe values determined using Mössbauer spectroscopy and electron energy-loss spectroscopy (EELS) are in good agreement. Fe3+/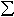 Fe shows a nearly linear dependence on mol% Al2O3 in (Mg,Fe)(Si,Al)O3 perovskite that is relatively independent of oxygen fugacity (see Annual Report 1997).
Fe shows a nearly linear dependence on mol% Al2O3 in (Mg,Fe)(Si,Al)O3 perovskite that is relatively independent of oxygen fugacity (see Annual Report 1997).
To investigate the substitution mechanisms in (Mg,Fe)(Si,Al)O3 perovskite, we combined chemical analyses from the electron microprobe with Fe3+/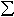 Fe determined by Mössbauer spectroscopy to obtain the total amounts of Fe2+ and Fe3+. With the assumption of 2 cations per formula unit, the oxygen concentration could then be calculated. Figure 3.2-4 illustrates the experimental data plotted on a ternary concentration diagram where vectors indicating the three substitution mechanisms are shown. Nearly all compositions fall between vectors A and B, indicating the presence of oxygen vacancies in (Mg,Fe)(Si,Al)O3 perovskite. Further evidence for oxygen vacancies comes from Mössbauer data which show the presence of tetrahedrally-coordinated Fe3+ (see following sections).
Fe determined by Mössbauer spectroscopy to obtain the total amounts of Fe2+ and Fe3+. With the assumption of 2 cations per formula unit, the oxygen concentration could then be calculated. Figure 3.2-4 illustrates the experimental data plotted on a ternary concentration diagram where vectors indicating the three substitution mechanisms are shown. Nearly all compositions fall between vectors A and B, indicating the presence of oxygen vacancies in (Mg,Fe)(Si,Al)O3 perovskite. Further evidence for oxygen vacancies comes from Mössbauer data which show the presence of tetrahedrally-coordinated Fe3+ (see following sections).
The compositions of (Mg,Al)(Si,Al)O3 perovskite include those that are relevant for the lower mantle perovskite phase, since the latter coexists with ferropericlase (Mg,Fe)O. The nature of the vacancies, whether they are isolated or clustered into chains or sheets, as well as the effect of pressure, temperature and oxygen fugacity could potentially affect physical properties such as electrical conductivity (see below) and elasticity.
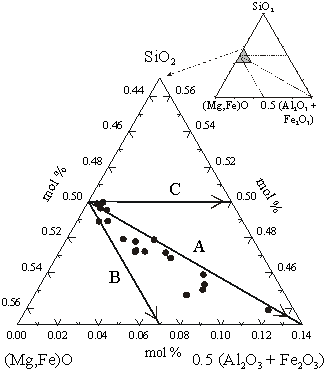 |
Fig. 3.2-4: Ternary concentration diagram showing the compositions of (Mg,Fe)(Si,Al)O3 perovskites. Vectors corresponding to the substitution mechanisms are shown. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page