

In the preceding section, evidence has been presented that silicate perovskites of the lower mantle might be oxygen-deficient. In order to study the effects of such oxygen defects on physical and chemical properties of perovskite-structured phases in general, we have embarked on the investigation of the system CaTiO3 - CaFeO2.5 (cf. Annual Report 1998). It provides a particularly suitable model system to address these questions: it exhibits the full range of possible defect concentrations, samples can be prepared at 1 bar under well-defined oxygen fugacities, interpretation of Mössbauer spectra is fairly straightforward due to the absence of Fe2+, electrical conductivity may be measured as a function of fO2 to determine the conduction mechanism, and finally these compounds are stable under the electron beam so that their mesoscopic structures may be determined. Among the structural phenomena to be described below are short-range and long-range order of oxygen vacancies, cation order-disorder, and displacive phase transitions, which all influence physical properties. As a reference for the all of which following sections, the phase diagram at 1 bar (cf. Annual Report 1998) is reproduced here again.
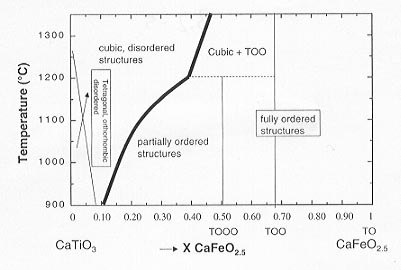 |
Fig. 3.2-5: Phase diagram of the solid solution CaTiO3-CaFeO2.5 as a function of temperature and composition. Symbols TO, TOO and TOOO indicate the sequence of tetrahedral (T) and octahedral (O) layers in the ordered structures. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page