

The ability of the perovskite structure to incorporate more than half of the stable elements in the periodic table in its crystal structure leads to a wealth of interesting properties, including many that involve a departure from the general formula ABO3. Indeed the most abundant mineral in the Earth's mantle, (Mg,Fe)(Si,Al)O3 perovskite, appears to contain significant numbers of oxygen vacancies (this Report). In a continuing study the system CaTiO3-CaFeO2.5 has been used to investigate the nature of oxygen vacancies and their relation to physical and chemical properties. Here, we investigate the nature of short-range ordering in the cubic, disordered phases in the system.
Samples were synthesised from mixtures of CaCO3, TiO2 and Fe2O3 and annealed at temperatures from 900-1320°C for periods ranging from 40 to 1660 h at controlled oxygen fugacity. Samples were drop quenched and examined ex-situ by electron microprobe, X-ray diffraction, Mössbauer spectroscopy and transmission electron microscopy. Electrical conductivity measurements were carried out on separate samples synthesised in a similar manner.
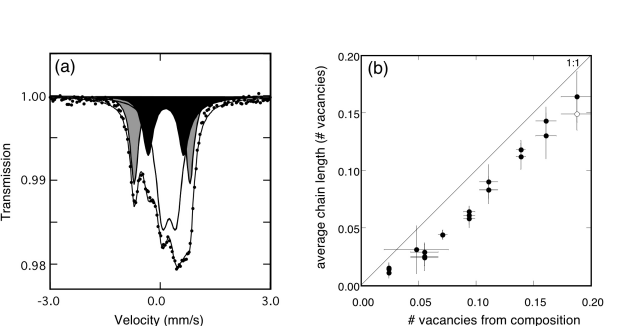
Mössbauer spectra show that all iron occurs as Fe3+, and that it is located in three different sites: octahedral, tetrahedral and pentacoordinated (Fig. 3.2-6a). The latter two sites arise as a result of oxygen vacancies in the lattice, where pentacoordinated Fe3+ has 1/2 vacancy per site, and tetrahedral Fe3+ has one vacancy per site. We can count the number of oxygen vacancies associated with the Fe3+ sites on this basis, and compare it with the number calculated from the constraint of charge neutrality (Fig. 3.2.-6b). There is a significant departure from the 1:1 relation, which can be interpreted as (1) a systematic error in the Mössbauer data, or (2) a failure to account for all oxygen vacancies. We have ruled out the first possibility, and so conclude that oxygen vacancies must also be associated with some of the Ti sites. This is the first time that evidence for non-octahedral Ti has been presented for this system.
 |
|
Fig. 3.2-6a: Mössbauer spectrum of CaFe0.3Ti0.7O2.85 at room temperature showing doublets corresponding to octahedral Fe3+ (unshaded), tetrahedral Fe3+ (grey) and pentacoordinated Fe3+ (black). The relative areas are roughly proportional to the relative abundances. |
Fig. 3.2-6b: Comparison of the number of oxygen vacancies per formula unit calculated from the Mössbauer data with the number calculated from the chemical composition based on charge neutrality constraints. The systematic offset from the 1:1 line is likely due to the presence of a small amount of Ti on non-octahedral sites. |
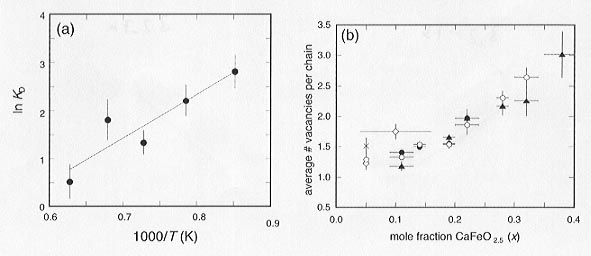
We assume that Ti is most likely found on pentacoordinated sites, which follows from the sequence of substitution of Fe3+ in the CaTiO3 lattice (see below). We have calculated the number of pentacoordinated Ti (PTi) atoms that are required to fit the 1:1 relation in Figure 3.2-6b, and found that there is a consistent trend in PTi with both temperature and composition: PTi decreases with increasing number of oxygen vacancies, and decreases with increasing temperature. The latter is illustrated in Figure 3.2-7a, where one expects a linear relation for Arrhenius behavior.
We can construct a model for the short-range ordering of oxygen vacancies in the cubic, disordered phase of the CaTiO3-CaFeO2.5 system. It is based on the model already described (Annual Report 1998) and incorporates new results from the present work. Starting from the CaTiO3 endmember, Ti on octahedral sites is replaced by Fe3+, forming oxygen vacancies to balance charge. The introduction of the first two Fe atoms results in the formation of two pentacoordinated sites and a monomer (a single oxygen vacancy), where the pentacoordinated sites would be occupied by Fe and Ti on the basis of entropy considerations, and the other Fe atom would replace Ti on an octahedral site. The presence of pentacoordinated Ti is consistent with our results described above. A dimer of oxygen vacancies can be formed with the substitution of two more Fe atoms, forming a tetrahedral site plus the two original pentacoordinated sites. As for the monomer case above, one Fe atom would likely be associated with the oxygen vacancy (in this case the tetrahedral site), while the other would replace Ti on an octahedral site. A trimer can be formed with the substitution of two more Fe atoms, and as above, one Fe atom would be associated with the new tetrahedral site, while the other would replace Ti on an octahedral site. In this way the proportion of Fe on octahedral sites should remain roughly 50% of the total number of sites occupied by Fe, which is consistent with our Mössbauer data.
The addition of further oxygen vacancies would increase the number of tetrahedral sites while the number of pentacoordinated sites remains constant (one at each end of the chain). It is straightforward to calculate the average number of vacancies per chain from the relative proportions of tetrahedral and pentacoordinated sites, and results show a nearly linear increase in chain length with increasing Fe concentration (Fig. 3.2-7b). Since the minimum number of vacancies per chain is one, samples with low iron concentration at high temperature must contain a high proportion of monomers.
These results are consistent with electrical conductivity measurements, which show that addition of Fe3+ to CaTiO3 initially increases ionic conductivity due to the increasing numbers of charge carriers, but that the effect is moderated at higher oxygen vacancy concentrations due to the increasing length of vacancy chains, which decreases the mobility of oxygen anions.
 |
|
Fig. 3.2-7a: Variation of ln KD [where KD = (OTi / PFe)/(PTi / OFe)] with inverse temperature, averaged over all compositions. The solid line indicates a linear fit to the data. |
Fig. 3.2-7b: Compositional variation of the average number of oxygen vacancies per chain calculated from the site abundance data assuming that Ti is distributed over both octahedral and pentacoordinated sites. Symbols indicate the annealing temperatures of the samples: 900°C (crosses); 1000°C (open diamonds); 1100°C (solid circles); 1200°C (open circles); and 1320°C (solid triangles). |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page