

Oxygen vacancy ordering in the system CaTiO3-CaFeO2.5 changes from isolated defects at low concentrations and high temperatures (cf. section 3.2.h, this report) to clusters of finite chains to complete ordering into defect layers and then ordering of such layers into regular sequences (cf. Fig. 3.2-5). An instructive example for ordering of vacancies is the structure of the endmember brownmillerite (CaFeO2.5) containing infinite chains of vacancies arranged in layers with pure tetrahedral (T) and octahedral (O) coordination of Fe3+ (TO structure).
Using transmission electron microscopy (TEM), we observed a similar ordering behavior for samples with lower mole fractions of CaFeO2.5. Samples with 0.1 < x < 1 have been annealed in the fields of partially and fully ordered structures (see phase diagram Fig. 3.2-5). The degree of ordering, the periodicity of TO sequences and the type of microtextures developed depend on annealing temperature, annealing time, and vacancy concentration.
Samples with a low mole fraction of CaFeO2.5 (x = 0.2) show a domain microtexture with average domain sizes on the order of 10 nm (Fig. 3.2-10). The domains have orthorhombic symmetry and occur in three mutually perpendicular orientations. In individual domains, high-resolution images show a large number of bright defect planes with a relative volume fraction of about 10%. This volume fraction is expected for almost complete accumulation of oxygen vacancies in planes with tetrahedral (T) coordination of cations. Strong streaks and the absence of superlattice reflections in selected area electron diffraction (SAED) patterns indicate a disordered TO sequence. Samples with a higher mole fraction (x = 0.35) still exhibit a domain microtexture but the domains are distinctly coarser (average size: 100 nm). Streaks in SAED patterns are less pronounced and lens-shaped superlattice reflections appear, indicating the increasing degree of ordering in the TO sequence with an approached periodicity of 18.9 Å. Due to further coarsening of domain sizes, samples with a mole fraction of x = 0.5 have developed a twin microtexture with almost complete ordering of TO sequences in individual twin domains. In agreement with previous TEM and X-ray studies we observed perfectly ordered TO structures with mole fractions of x 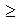 0.5. Altogether the TEM observations indicate that ordering in oxygen-deficient perovskite is a two-step process starting with the formation of infinite chains of oxygen vacancies that order into crystallographic planes and evolve finally to a fully ordered TO sequence.
0.5. Altogether the TEM observations indicate that ordering in oxygen-deficient perovskite is a two-step process starting with the formation of infinite chains of oxygen vacancies that order into crystallographic planes and evolve finally to a fully ordered TO sequence.
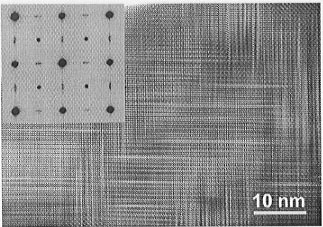 |
Fig. 3.2-10: High-resolution TEM image of partially ordered perovskite CaTi0.8Fe0.2O2.9. Defect planes composed of aligned bright dots are interpreted to represent layers in the structure with almost complete tetrahedral coordination of cations. The remaining regions represent layers with octahedral coordination of cations. The inset shows the corresponding electron diffraction pattern that represents a superposition of reflections from three sets of domains being mutually perpendicular. |
Complementary electrical conductivity measurements demonstrate the dramatic effect of oxygen vacancy ordering on bulk properties. For a sample with x = 0.3, the electrical conductivity decreases by more than one order of magnitude at a temperature of ca. 1150°C. This temperature coincides with the order-disorder boundary in the phase diagram CaTiO3 - CaFeO2.5. (cf. Fig. 3.2-5). Any interpretation of electrical conductivity data of the lower mantle, which is predominantly composed of perovskites, probably with some defects, therefore has to take this finding into account.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page