

Early in the history of the Earth, separation and segregation of Fe-Ni metal led to the formation of the Earth's core. During this separation siderophile elements in the silicate mantle would have been strongly partitioned into the separating core material. Thus the present concentrations of siderophile elements in the Earth's mantle contain information about the nature of the core forming process and two models have been proposed to explain their abundance: (a) Homogeneous accretion, where mantle siderophile element concentrations reflect a global core/mantle (metal/silicate) equilibrium; (b) Heterogeneous accretion or "late veneer" model, where siderophilic elements were replenished by a later stage accretion event after initial core formation finished.
In order to distinguish between the two models a knowledge of high pressure and temperature metal/silicate partition coefficients is required, in particular their dependence on pressure (P), temperature (T) and oxygen fugacity (fo2). Partition coefficients at high pressure are still not sufficiently well-known to firmly differentiate between the two models.
As shown in the 1998 Annual Report it is possible to qualitatively estimate the pressure dependence of metal/silicate partition coefficients (Kd) by determination of the partial molar volumes of all components participating in the corresponding distribution reaction. With the assumption of a constant pressure-independent volume change, the pressure dependence at fixed temperature of Kd can be calculated from
In the case of non-ideal mixing in silicate liquids a proper evaluation of the volume change ( V) requires the knowledge of partial molar volumes of metallic elements dissolved in silicate liquids.
V) requires the knowledge of partial molar volumes of metallic elements dissolved in silicate liquids.
We have, therefore, determined the partial molar volumes of Ga2O3 and GeO2 through density measurements of Ga- and Ge-bearing silicate melts using the double bob Archimedean method. Measurements on Ga- and Ge-bearing Na-disilicate (NS2) liquids (1998 Annual Report) were extended to an anorthite-diopside eutectic (AD) liquid. The results of these new measurements are shown in Figure 3.3-8, where densities are plotted as a function of temperature. In Figure 3.3-9 molar volumes calculated from the densities for all systems studied are plotted as a function of mole fraction of Ga2O3 and GeO2.
The mixing behavior of the molar volumes is linear in all systems except NS2-Ga2O3, where a strong non-linearity is observed. The non-linearity is likely due to a coordination change of Ga from 4-fold to 6-fold across the binary join, whereas the ideal mixing behavior in the AD-Ga2O3 system may reflect a single coordination state of Ga. We suggest that in the AD-system the tetrahedral position is already saturated with Al, preventing Ga from entering the tetrahedral sites and thus restricting Ga to the octahedral environment. The ideal mixing behavior with respect to volume for Ge is readily explained by its stereochemical similarity to Si which is replaced by Ge on the tetrahedral position for all Ge contents. Calculated volume changes of the exchange reaction for Ga and Ge between metal and silicate melt are given in Table 3-1.
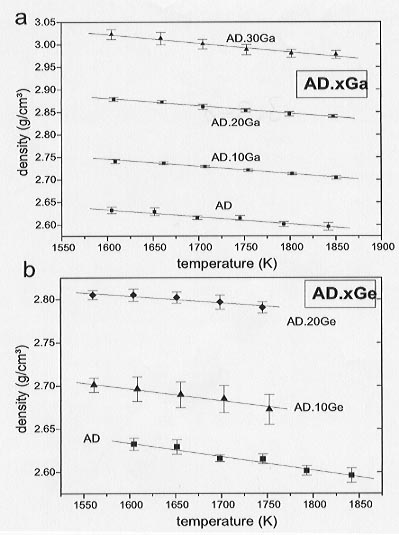 |
Fig. 3.3-8: Density versus temperature for the AD-systems.
a) AD-Ga2O3 ; b) AD-GeO2. |
Table 3-1: Calculated volume change at 1600 K
| NS2-systems | AD-systems | |
| ΔVGa-Fe (cm3/mole) | -5 ± 4 | 3.1 ± 0.3 |
| ΔVGe-Fe (cm3/mole) | -5.7 ± 0.4 | -5.9 ± 0.4 |
NS2 is a Na-disilicate melt and AD is an anorthite-diopside eutectic melt. The mixing behavior of the metal-phase is assumed to be ideal and therefore molar volumes of the pure molten metals were used.
A negative volume change of reaction leads to an increase in the siderophilic character of the element with increasing pressure. Therefore, pressure would favour the extraction of Ge from the mantle and a heterogeneous accretion model would be required to explain the present day mantle concentration. The same conclusion is reached for Ga in NS2-melts whereas for the AD-liquids (which are more representative of a mantle composition than NS2-liquids) Ga exhibits a decrease of siderophility with increasing pressure.
While the experimental results obtained in this study are only applicable for GeO2, at oxygen fugacities applicable to core formation GeO may also be present. Similarly, our extrapolations are only valid for Ge in 4-fold coordination as the partial molar volumes depend strongly on the coordination number. It is possible that an increase in coordination could occur at higher pressure, which would result in a decrease in the partial molar volume and therefore a decrease in the siderophilic character of Ge with pressure. The results of this study may be considered as a first step towards a better understanding of the effects of pressure on the partitioning of siderophile elements between metal and silicate.
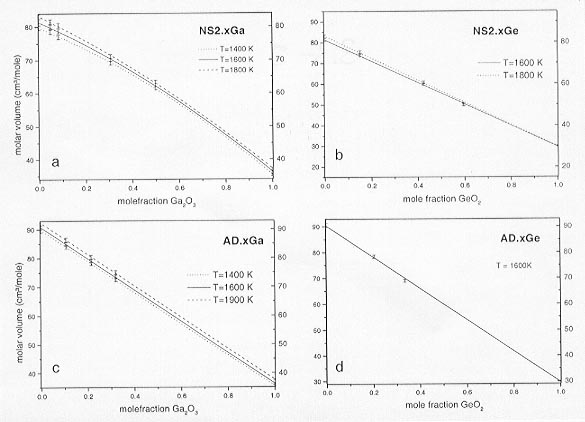 |
Fig. 3.3-9: Molar volume versus mole fraction of siderophile component.
a) Ga-bearing NS2-liquids; b) Ge-bearing NS2 liquids; c) Ga-bearing AD-liquids; d) Ge-bearing AD-liquids. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page