

Determination of the phase relations in the Mg2SiO4-Fe2SiO4 system at high pressure and temperature is critical to our understanding of the mineralogy of the upper mantle and transition zone. Phase diagrams for this system at high pressure and temperature have been produced from multianvil phase equilibria experiments but difficulties in the calibration of pressure can result in significant uncertainties in phase boundaries and widths of coexisting loops. The topology of such loops will, to some degree, control the depth and sharpness of the 410 and 660 km seismic discontinuities.
Thermodynamic calculations have been used in the past to produce internally consistent phase diagrams by combining phase equilibria and thermochemical data. A thermodynamic treatment of the Mg2SiO4-Fe2SiO4 system requires information on the activity-composition relationships of the solid solutions. In this study experiments were performed to determine equilibrium Fe-Mg partitioning between the high-pressure olivine polymorphs and magnesiowüstite solid solutions. Using activity-composition data for the magnesiowüstite solid solution then allows the calculation of activities for wadsleyite and ringwoodite from the determined partition coefficients. Magnesiowüstite is, in effect, being used as a compositional probe to compare partitioning between the silicate phases that do not coexist over very large pressure intervals. As the Fe-Mg partitioning is not particularly sensitive to pressure or temperature the shapes of coexisting regions of the phase diagram can be calculated without incorporating the pressure uncertainties inherent in phase equilibria determinations. In addition, different capsule materials can be used to asses the effect of oxygen fugacity on the partitioning and consequently on the phase diagram.
Starting compositions of olivine and magnesiowüstite are ground together and loaded into multi-chamber metal capsules each with four or six 0.3 mm-diameter sample holes. Capsules were spark eroded from rods of either Fe, Mo or Re. For experiments in Fe capsules 5 mole % Fe is also added to the starting compositions. Two capsules can be placed inside a single 10/5 or 7/3 multianvil assembly, as shown in Figure 3.3-10, such that 8 or 12 different compositions can be tested in a single experiment. Experiments are performed between 1000 and 1600°C and from 15 to 25 GPa for durations between 10 and 96 hours. Final compositions of the silicate and oxide phases are approached from starting compositions with both higher and lower Fe contents in order to demonstrate equilibrium. In some experiments starting compositions are previously transformed to the high pressure polymorphs. Sample compositions are analysed using electron microprobe and the identity of the silicate phases can be determined by Raman spectroscopy.
Partitioning relations for ringwoodite determined at Fe saturation are shown in Figure 3.3-11. The pressure and temperature effects on the partitioning are small over the range of experimental conditions and almost within experimental uncertainty. Activity-composition relations for the solid solution can be determined from those of the oxide using the Fe-Mg partitioning data. For experiments performed at Fe saturation the oxygen fugacity of each composition can be measured using the 2Fe + O2 = 2(Fe,Mg)O redox reaction.
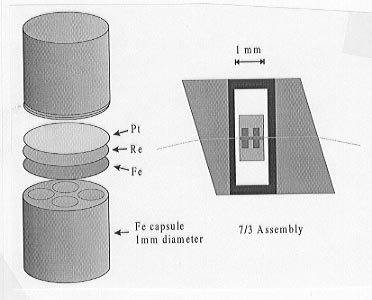 |
Fig. 3.3-10: Fe capsules and 7/3 multianvil assembly. |
Results obtained in this study for Fe and Re capsules show that most previous studies have been performed at moderately high oxygen fugacity, close to Re-ReO2. The results also indicate that the ringwoodite and wadsleyite solid solutions are more ideal under more reduced conditions, suggesting that the widths of the 410 and 660 km discontinuities may be sensitive to oxygen fugacity.
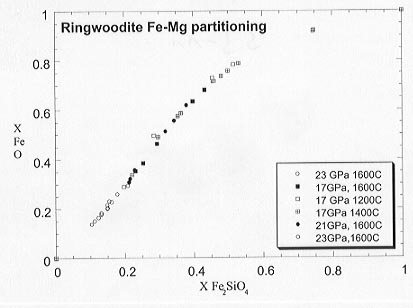 |
Fig. 3.3-11: Results of Fe-Mg partitioning between ringwoodite and magnesiowüstite between 17 and 23 GPa.
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page