

Recent geochemical models of the formation of the Earth's metallic core suggest that liquid metal segregated from silicates in a magma ocean 700-1000 km deep and equilibrated at the base of this magma ocean at a pressure of 25-30 GPa. The liquid metal fraction then sank as large diapirs through the lower mantle without significant chemical reequilibration at higher pressures. Such models can explain the present abundances of siderophile elements (e.g. Ni and Co) in the Earth's mantle on the basis of experimental studies of element partitioning at high pressures. These models imply that limited disequilibration reactions must have occurred during transport of metal through the lower mantle. A further implication is that the metallic core would not be in equilibrium with the lower mantle and that disequilibrium reactions could still be occurring at the core-mantle boundary, depending on their kinetics. Such reactions might involve siderophile elements and also light elements such as oxygen and silicon.
Preliminary experiments have been performed at 10 GPa and 1900°C in a multianvil apparatus to study the kinetics of reactions between magnesiowüstite and Fe-rich metal liquids that are supersaturated in oxygen. Mixtures of Fe, Fe3O4 and Ni (˜6 wt% oxygen) are contained in an MgO capsule in a multianvil sample assembly. The latter is fabricated from an MgO single crystal in order to ensure that chemical exchange is controlled by lattice diffusion in the oxide. At the experimental conditions, the metal is liquid and reacts with the MgO. Due to supersaturation in oxygen, reaction initially involves the growth of a FeO-rich rim between the MgO and the liquid metal, which consumes oxygen (Fig 3.3-12). With increasing time,
 |
Fig. 3.3-12: Reflected light micrograph of a quenched high-pressure sample. The central light region consists of metal that contains small exsolved oxygen-rich blobs that formed during quenching. The light grey (˜100 µm-wide) rim adjacent to the metal is FeO-rich magnesiowüstite that precipitated from the metal early during the experiment and then partially equilibrated with the surrounding MgO by interdiffusion (see Fig. 3.3-13). The cracks in the sample (black) form during decompression and sample preparation. |
(1) this rim partially equilibrates with the MgO through Fe-Mg interdiffusion (Fig. 3.3-13), and (2) the composition of the oxide in contact with the liquid metal becomes increasingly rich in MgO. These results indicate that the oxygen fugacity, which controls the partitioning of Fe and Ni between the metal and oxide, decreases with time as the oxygen content of the metal decreases. A fit to our earlier experimental data on the solubility of oxygen in liquid metal (see Annual Report 1998) can be used to quantify this effect. The evolution of the Fe-Mg concentration profile in the oxide can be modelled using Fe-Mg diffusion coefficients for magnesiowüstite that are a strong function of composition. The required diffusion coefficients are consistent with those determined experimentally for magnesiowüstite at 1 bar (see 1998 Annual Report), with a small correction for the effect of pressure. Further experiments are being performed in order to quantify the boundary conditions and enable a reaction/diffusion model involving a moving interface and changing interface composition to be developed. The results will be used to understand the nature and extent of reactions between liquid metal diapirs and silicates and oxides in the lower mantle during the early history of the Earth.
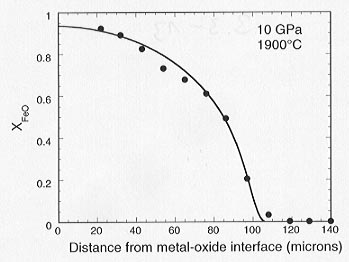 |
Fig. 3.3-13: Compositional profile in magnesiowüstite that developed adjacent to liquid Fe-Ni-O metal where XFeO = FeO/(FeO+MgO). The solid line shows a calculated profile based on a model of Fe-Mg interdiffusion between a FeO-rich rim and the MgO capsule. The experimental run time was about 10 seconds. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page