

Diffusion in silicate minerals is so slow, that it is often not possible to study low-temperature diffusion processes or short duration diffusion events. We have carried out experiments on olivine to generate compositional profiles under controlled conditions and used analytical TEM (with EDX) to measure diffusion profiles on the sub-micron scale. Our objective was to test the feasibility of this method for the measurement of diffusion coefficients and having demonstrated this, to extend the available data set for olivine to conditions that were previously not accessible.
Diffusion anneals were performed with diffusion couples consisting of two single crystals of olivine at temperatures above 900°C, and a thin fayalitic film on olivine at temperatures between 800 and 900°C. The latter technique was developed during the course of this study to overcome the problem of bonding of single crystals at low temperatures.
Figure 3.3-14 shows the Mg-Fe interdiffusion coefficients obtained from the single crystal as well as the thin film experiments. Data obtained using conventional profile measurements with an electron microprobe are also shown for comparison. It can be seen from Figure 3.3-14 that both methods yield the same activation energy within experimental errors. Considering all data obtained in this study, there is no indication of a change in activation energy down to 800°C as has been reported in the literature. Other significant findings of this study are:
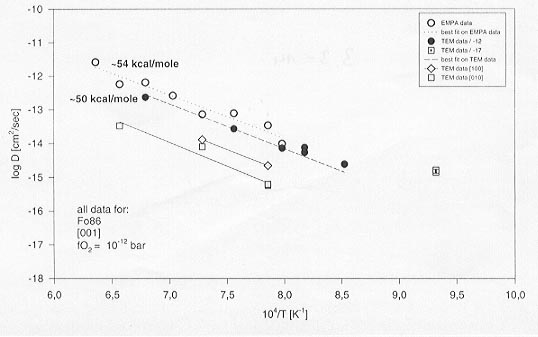 |
Fig. 3.3-14: Arrhenius diagram showing diffusion coefficients for Mg-Fe interdiffusion in olivine between 1300 and 800°C at room pressure.
All data was obtained from single crystal olivine or thin-film diffusion couples and was measured by either TEM-EDX or EMPA. |
(i) The diffusion coefficients for the different crystallographic directions are in the order D[001]>D[100]>D[010]. Although diffusion along [100] and [010] directions is slower, the activation energy seems to remain the same as along [001], in contradiction to some earlier reports.
(ii) TEM images of samples from anneals at the same temperature but different experimental times (4 min -100 hours) show that the low temperature or "short duration" samples have a high denisty of planar and/or line defects near the interface region. The same diffusion coefficient, however, was calculated from the resulting diffusional profiles, which ranged from 1 µm to several tens of micrometers in length. As interdiffusion of Mg:Fe in olivine occurs by a vacancy mechanism, it can be concluded that point defect equilibrium in the interface region is obtained rapidly at 1200°C . The planar/line defects present at the interface take longer to anneal out, but appear to have had no measurable influence on the diffusion rate.
(iii) Anneals carried out at the same temperature but different oxygen fugacities show that Fe-Mg diffusion rates depend on oxygen fugacity, as found previously, but that this dependence appears to decrease with temperature such that below 900°C this dependence is probably very small.
It can be seen from this investigation that using TEM EDX to measure sub-µm diffusional profiles has a great potential for further diffusion studies where it is not possible to produce long compositional profiles due to various experimental difficulties (e.g. buffered high-P experiments). This method provides a unique opportunity to investigate the relation between defects and diffusional processes. In further studies it is planned to measure diffusion coefficients in clinopyroxene where, even at high temperatures, diffusion rates are very slow.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page