

Preliminary in-situ Raman experiments using an externally heated diamond-anvil cell showed that H4SiO4 is the dominant silica species in aqueous fluids in equilibrium with solid quartz. The successful installation of a new sophisticated Raman spectrometer (DILOR XY) equipped with a large charge-coupled device (CCD) detector and confocal entrance optics enabled these experiments to be extended to larger frequency, temperature and pressure ranges.
Figure 3.4-1 shows typical unpolarized Raman spectra of aqueous fluid in equilibrium with quartz from 600 to 900°C for a bulk density of water of 0.94 g/cm3. This corresponds to pressures between 8.8 kbar at 600°C and 14 kbar at 900°C. Time series experiments showed that equilibrium concentrations are reached within a few minutes. Up to 600°C only one band is seen at 760 - 785 cm-1, which was previously assigned to the symmetric stretching vibration of H4SiO4 molecules in agreement with accepted models of silica speciation in water.
At temperatures above 600°C additional bands (marked by arrows in Fig. 3.4-1) start to develop in the Raman spectra. These broad bands at 630, 920, and 230 cm-1 must be due to an additional silicate species coexisting with H4SiO4. The most simple silica species aside from orthosilicic acid would be pyrosilicic acid H6Si2O7, which could form according to the polymerization reaction 2 H4SiO4 = H6Si2O7 + H2O.
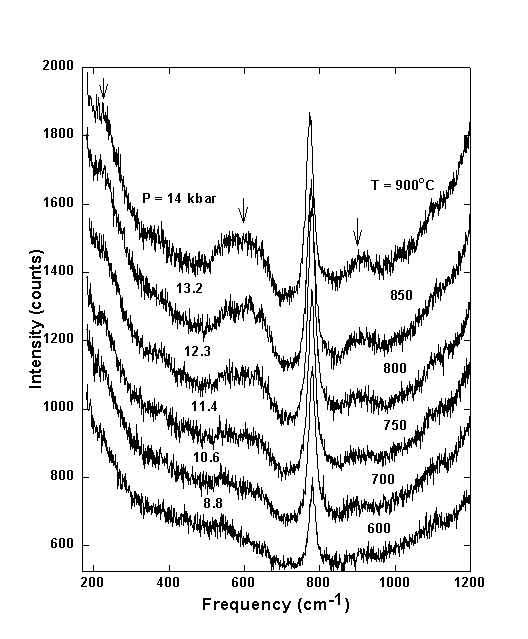 |
Fig. 3.4-1: Unpolarized Raman spectra of aqueous fluid in equilibrium with quartz. Individual spectra have been offset for clarity. |
Calculations of the normal frequencies, isotopic shifts and Raman intensities of H4SiO4, H6Si2O7, and partially deprotonated H4-nSiO4n- molecules have confirmed these assignments. They also indicate that at typical crustal pressures and temperatures the presence of high concentrations of ionized species in the solution can be ruled out.
The strongest band of the pyrosilicic acid H6Si2O7 molecules at about 630 cm-1 is much broader than the characteristic H4SiO4 band at about 780 cm-1 (Fig. 3.4-1). This is probably due to a distribution of H6Si2O7 dimers with different Si-O-Si bridging and dihedral angles and to the presence of higher chain-like condensation products.
In the low-temperature, low-pressure regime where only H4SiO4 species are observed the integral intensity I of the characteristic peak of the orthosilicic acid should be proportional to the molal concentration m of dissolved quartz. The later can be accurately calculated up to 900°C and 10 kbars using an empirical equation derived from available solubility data. Figure 3.4-2 shows a is the only species up to 500-600°C and 6-10 kbar pressures. The precise pressures and temperatures above which H6Si2O7 molecules start to appear depends on the density of the fluid.
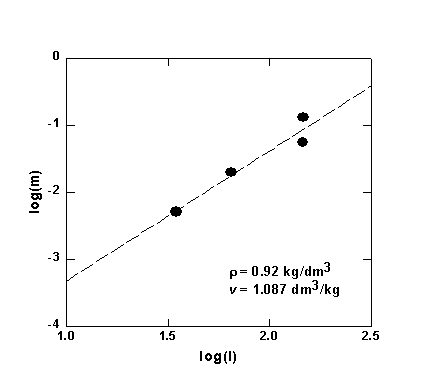 |
Fig. 3.4-2. Plot of the molal solubility of quartz in water (m) versus the intensity (I) of the Raman peak of H4SiO4 in hydrous solutions in equilibrium with quartz. |
At the P,T conditions of the upper mantle both H4SiO4 and H6Si2O7 species are present (Fig. 3.4-1). Above 750°C the intensities of the orthosilicic acid start to decrease at the expense of the intensity of the pyrosilicic acid. Using the integral intensities of the characteristic Raman bands, corrected by the Bose-Einstein thermal population factor, we have estimated the equilibrium constant K of the polymerization reaction 2 H4SiO4 = H6Si2O7 + H2O. The required scattering cross-sections were taken from our Raman intensity calculations. Figure 3.4-3 shows a typical log(K) plot for an agueous solution with bulk density 0.949/cm3. From this plot, it appears that with increasing temperature, polymerization increases. However, the data shown in this figure refer to an isochore. Accordingly, pressure increases simultaneously with temperature. The thermodynamic analysis of several experiments with different density demonstrates that polymerization is mainly caused by the increase of pressure, while the effect of temperature is small.
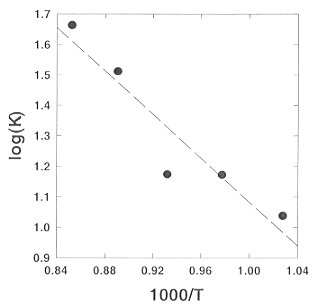 |
Fig. 3.4-3: Plot of the equilibrium constant K of the polymerization reaction 2H4SiO4 = H6Si2O7 + H2O versus 1/T for an isochoric experiment. Note that pressure increases with temperature. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page