

Due to the almost overwhelming complexity of pegmatite bodies, and the concentration of such elements as F, B, Sn, U, Th, Nb, Ta, Be, Li, Cs and the REE in such bodies, the study of pegmatites and their genesis has a long history. The most widely accepted model of pegmatite formation relies on a three-stage process:
- Crystallization from a hydrous silicate melt, producing anhydrous solid phases ± OH-bearing phases.
- Crystallization from the silicate melt and from a coexisting exsolved aqueous fluid, yielding giant textured pegmatite. Constituents are partitioned between these two phases, and these diffuse rapidly through the aqueous phase. The fluid also rises due to its low density, forming pods and similar structures in the upper level of the intrusion.
- Crystallization of the fluid, yielding a number of late stage products and mineral aggregates formed through the exchange of material between the fluid and previously formed minerals.
However, work performed in the last few years in Bayreuth has shown that coexisting melt and aqueous fluid will in fact form a single supercritical phase at high temperatures and pressures. As the presence of such components as F can alter the behavior of granitic melts this present study was designed to examine if supercritical behavior could be important in the petrogenesis of pegmatite bodies.
A reconnaissance study was performed on a glass composed of 66.5 wt% albite, 19.5 wt% NaF, 9.5 wt% B2O3 and 4.5 wt% water, synthesised at 2 kbars and 1100°C for three days in a rapid quench TZM autoclave. Several additional albitic glasses were synthesised containing 3.5 wt% water and 5 - 10 wt% of excess Na, F, B or Li at 1 kbar, 1100°C for seven days. All the experiments were carried out using a hydrothermal diamond-anvil, cell which allows for the direct observation of supercritical behavior in-situ at temperature and pressure.
The results of the reconnaissance study and the results for pure albite are compared in Figure 3.4-4. As can be seen, the presence of additional Na, F, and B has a dramatic effect on the supercritical curve of albite. At reasonable pressures for the emplacement of pegmatites, the critical temperature for the system has been lowered by at least 600°C, and the curve has a different shape.
The results of the experiments on the Na, F, and B bearing albite glasses are shown in Figure 3.4-5. All three of the additional components cause a shift in the critical curve for pure albite. The presence of F and B lowers the critical temperature by around 150°C. The presence of excess Na also has a strong effect, not only lowering the critical temperature, but changing the slope of the critical curve.
Combined with the reconnaissance study, it is clear that the effect of the single additional components are roughly additive, and that the presence of more than one will produce a much larger effect on the critical behavior of albite. These results are not only important for the formation of pegmatites, but may also be useful in the petrogenesis of other granitic systems.
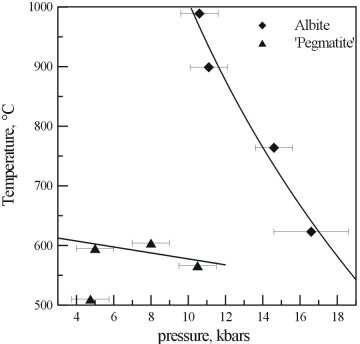 |
Fig. 3.4-4: A comparison of the critical curves obtained from the reconnaissance study with albite containing excess Na, B, and F, and that of pure albite. |
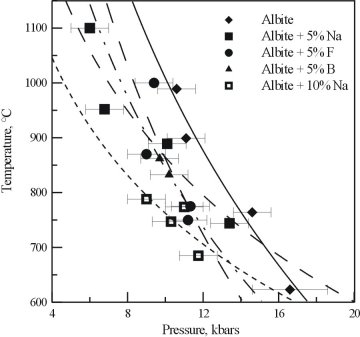 |
Fig. 3.4-5: Critical curves obtained for albites containing excess Na, F and B. Also shown is the albite curve from Figure 3.4-4. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page