

Sulfur compounds, in particular SO2 and H2S, are important constituents of volcanic gases. The injection of sulfur dioxide into the stratosphere is known to be responsible for the global cooling observed after major volcanic eruptions. The composition of the gases released during an explosive eruption is determined by the phase equilibria between fluid, silicate melt, and crystalline phases in the magma chamber several kilometers below the volcano. It is well established that the oxygen fugacity in the magma chamber has a strong effect on the distribution of sulfur between the silicate melt and solid phases. At low fo2, most magmas crystallize pyrrhotite (FeS) upon cooling, while at high fo2, anhydrite (CaSO4) may precipitate. At intermediate oxygen fugacities, no sulfur-bearing minerals are stable, and accordingly, such conditions are very favorable for releasing massive amounts of sulfur to the atmosphere.
Ultimately, the amount of sulfur released in an eruption is determined by the partitioning of sulfur species between a hydrous fluid and the silicate melt. However, no direct measurements of the influence of oxygen fugacity on the fluid/melt partition coefficient Dfluid/melt of sulfur were available. Accordingly, a series of partitioning experiments was conducted in the system haplogranite-H2O-S at 2 kbars and 850°C, with variable oxygen fugacities ranging from the Co-CoO buffer to the Fe3O4-Fe2O3 buffer. All experiments were carried out in rapid-quench autoclaves, either with bombs made out of the Ni-Cr alloy Nimonic 105 and water as pressure medium, or with TZM bombs pressurized by argon. Sulfur contents in quenched glasses were measured by electron microprobe; the fluid composition was calculated by mass balance.
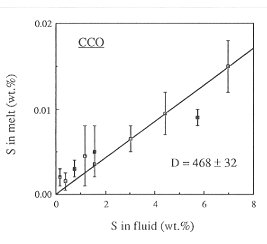
Figure 3.4-6 shows the fluid/melt partitioning of sulfur for the oxygen fugacity of the Co-CoO buffer and for an oxygen fugacity 0.5 log units above the Ni-NiO buffer. A drastic effect of redox conditions on the behavior of sulfur is obvious. Under reducing conditions, Dfluid/melt is 468, one of the highest fluid/melt partition coefficients ever measured for any element. With increasing oxygen fugacity, Dfluid/melt decreases by about one order of magnitude. At an oxygen fugacity close to the Ni-NiO buffer, Dfluid/melt is 47. Upon further increase in fo2 to the Fe3O4-Fe2O3 buffer, the partition coefficient remains nearly unchanged.
 |
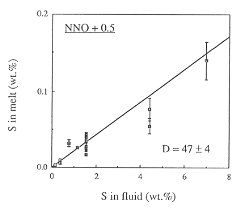 |
Fig. 3.4-6: Partitioning of sulfur between haplogranite melt and aqueous fluid at 2 kbars, 850°C and the oxygen fugacity of the Co-CoO buffer (CCO) or 0.5 log units above the Ni-NiO buffer (NNO + 0.5). |
|
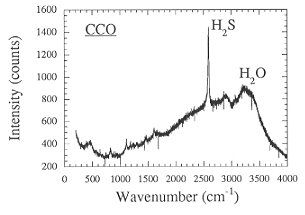
The strong effect of redox conditions on the behavior of sulfur suggests some change in speciation. This was verified by measuring the Raman spectra of quenched fluid trapped as inclusions inside the glassy run products (Fig. 3.4-7). Apparently, sulfur is present as H2S under reducing conditions, while SO2 dominates at more oxidizing conditions.
The data presented here demonstrate that a small trace of fluid in a magma chamber can effectively extract most of the sulfur out of a large magma reservoir. This effect will be particularly pronounced at low oxygen fugacities.
 |
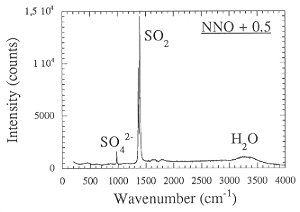 |
Fig. 3.4-7: Raman spectra of aqueous fluids quenched from 2 kbars, 850°C and the oxygen fugacity of the Co-CoO buffer (CCO) or 0.5 log units above the Ni-NiO buffer (NNO + 0.5). |
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page