

Water has a substantial effect on the properties of silicate melts. However, the exact interaction mechanism between water and the structural units in aluminosilicate melts and glasses is still a matter of some debate. In order to address this, Raman spectra have been obtained from a series of rhyolite and basalt glasses with varying water contents.
Figure 3.4-10 shows the unpolarised spectra obtained from the rhyolite samples, scaled in a way that the low-frequency bands at around 480 cm-1 have the same intensity. The spectra are typical for highly polymerized glasses. Systematic changes are observed with increasing total water content (TWC). There is a narrowing of the strong low-frequency band at about 480 cm-1, which arises mainly from bending and rocking motions of the T-O-T linkages, indicating that the T-O-T bond angle distribution becomes more narrow with increasing TWC. The peak at about 580 cm-1, which is usually assigned to 3-membered rings, disappears in the samples with TWC above 4 wt%. The intensity of the 800 cm-1 band, which arises from complex motions of the silicon atoms in Q4 units, decreases systematically with increasing TWC, which indicates that the structure becomes more depolymerised. This is further confirmed by the increase of the intensity of the 910 cm-1, band which is commonly assigned to T-OH bending vibrations. Subtle changes in the high-frequency band, which arises from symmetric Si-O stretching-type vibrations, indicate changes in the Q-species distribution without significant increase of the Q3 species. The O-H stretching band extends from 2900 to 3700 cm-1 and shows a linear increase in intensity with increasing TWC. The O-H bands indicate that there is a broad distribution of hydrogen-bond lengths in all samples regardless of the OH-H2O distribution, which means that in all samples both the OH groups and H2O molecules participate in hydrogen bonding. With increasing TWC there is a slight shift of the maximum of the O-H band at about 3550 cm-1 to lower frequencies which indicates an increase of the strength of the hydrogen bonding.
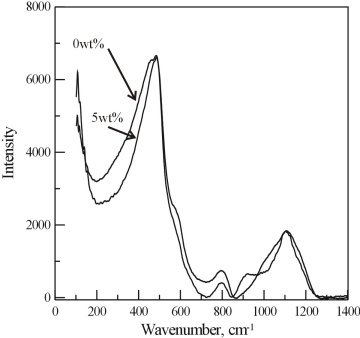 |
Fig. 3.4-10: Raman spectra of rhyolite glasses containing 0, 3, 4, and 5 wt% H2O. |
The unpolarised Raman spectra shown in Figure 3.4-11 indicate that the structure of the hydrous basalt glasses is much more depolymerised. The intensity in the 200 - 400 cm-1 range is significantly increased compared to the Raman spectrum of the rhyolite glasses, due to the increased number of non-bridging oxygen atoms. The T-O-T band is shifted to higher frequencies and is broader, indicating that the T-O-T bond angle distribution in basalt glasses is broader. With increasing TWC, the intensities around 200-400 cm-1 increase. At the same time, a slight narrowing of the low-frequency band is observed, similar to the hydrous rhyolites. The intensity of the 800 cm-1 band is much weaker than in the rhyolite glasses and with increasing water content it decreases further. This again is evidence for a much more depolymerized glass structure. The high frequency band at about 1000 cm-1 suggests the presence of mostly Q2 and Q3 species. With increasing TWC above 3 wt%, the relative intensity of the 1000 cm-1 band decreases at the expense of increased intensity around 850 cm-1. This suggests that the relative amount of Q3 species decreases probably at the expense of Q1 species. The high-frequency band in the basalt glasses is broader than in the rhyolite glasses, which implies larger bond-length and bond-strength disorder in the basalt glasses. A new peak at about 650 cm-1 is observed in the basalt glasses, which becomes less intense, shifts to slightly higher frequencies and becomes more narrow with increasing TWC. Polarized measurements show that this peak possesses a low depolarization ratio and thus arises from symmetric-type vibrations. Although the behavior of this peak implies changes in the structure, additional studies are necessary in order to elucidate the origin of this peak in basalt glasses. Finally, the shape of the O - H stretching bands in the hydrous basalt glasses is very similar to those in the rhyolites, but the band extends from 2700 to 3700 cm-1. This implies that the environment of O - H groups in rhyolite and basalt glasses is generally similar, and that depolymerisation increases hydrogen bond strength.
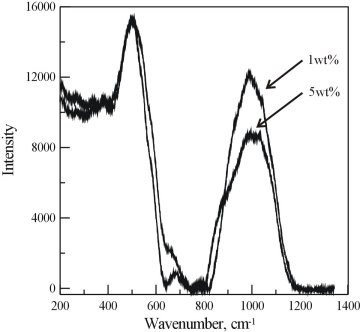 |
Fig. 3.4-11: Raman spectra of basalt glasses containing 1, 2, 3 and 5 wt% H2O. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page