

The kinetics of hydrogen diffusion in single crystals of orthopyroxene were investigated for diffusion parallel to the [100], [010] and [001] crystallographic directions during dehydration and hydrogenation. Xenolithic crystals from San Carlos, Arizona, contained on average 5.3 wt% FeO and 2.4 wt% Al2O3, and the gem-quality crystals from Sri Lanka contained 4.5 or 8.5 wt% FeO, and 2.1 or 3.6 wt% Al2O3. Dehydration was performed in a 1 bar gas-mixing furnace at temperatures between 800 and 1100°C, using mixtures of CO and CO2 to maintain the oxygen fugacities in the range from 10-9 to 10-15 bar. Hydrogenation was performed in a piston-cylinder apparatus at 1 GPa within the same range of temperatures, using welded platinum capsules to contain the samples, water, an olivine substrate and the nickel/nickel oxide oxygen buffer. Hydroxyl concentration profiles, such as those in Figure 3.4-15, were measured across the polished central sections of the samples using polarized FTIR spectroscopy with the electric field vector E oriented parallel to the c crystallographic direction, the direction in which hydroxyl dipoles in clinopyroxene are primarily oriented. Hydrogen diffusivities were obtained by fitting a theoretical law for diffusion in a finite slab to the measured hydroxyl concentration profiles.
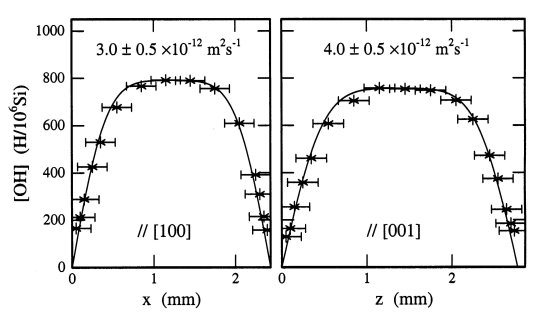 |
Fig. 3.4-15: Hydrogen concentration profiles parallel to [100] and [001] for sample OPX 11-2, dehydrated at 1 bar, 1000°
C and an oxygen fugacity of 10-9 bar for 5 hours. The diffusivities, indicated by the solid lines, were calculated from fits to the experimental data. |
During dehydration, hydrogen diffusion was found to be anisotropic in San Carlos enstatite, and isotropic in the Sri Lankan samples, with more rapid hydrogen diffusion occurring in the Sri Lankan samples that contained greater amounts of iron. Hydrogen diffusivities are D[001] = 1.63 x 10-5 exp[-161 ± 17 kJ/mol/RT] m2s-1 for San Carlos enstatite parallel to c, the fastest direction of diffusion, DOPX14 = 9.12 x 10-5 exp[-195 ± 48 kJ/mol/RT] m2s-1 for Sri Lankan orthopyroxene containing the lowest amounts of iron, and DOPX11 = 91.4 exp[-326 ± 27 kJ/mol/RT] m2s-1 for Sri Lankan orthopyroxene containing the greatest amounts of iron. The mechanism of hydrogen diffusion in orthopyroxene is believed to be a coupled redox reaction that incorporates (or releases) hydrogen with a concurrent change in the oxidation state of iron. In all cases, hydrogen diffusivities are approximately 30-100 times lower than those determined for San Carlos olivine and Jaipur diopside.
Measurements of hydroxyl concentrations across untreated crystals of San Carlos enstatite showed no spatial variability, even though hydrogen must have been mobile during ascent from the mantle source region. Thus, it appears likely that the hydrogen content of the natural samples equilibrated rapidly with that in the surrounding melt during transport, and likely represents partitioning between the crystals and melt at some relatively shallow depth rather than the water content of the source region.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page