

The properties of silicate melts are very pressure dependent due to the continuous transformation of a three-dimensional tetrahedral framework at ambient pressure to a liquid composed of increasing amounts of Si4+ and Al3+ cations in higher coordination states as pressure increases. Variation in density and transport properties in response to pressure-induced coordination changes is of paramount importance to processes such as magma ascent and emplacement, crystal nucleation, growth and segregation. In order to examine the effects of pressure on the structure of silicate liquids, we have synthesized a series of glasses with composition 44CaO + 12Al2O3 + 44SiO2 at pressures as high as 10 GPa using a multianvil apparatus for an investigation by x-ray absorption spectroscopy. Measurements of the Al, Si, and Ca K-edges of the glasses were carried out at SSRL (Stanford, California) on the SB03-3 beamline, equipped with a JUMBO double-crystal monochromator. XANES spectroscopy is particularly useful in the structural characterization of amorphous materials because of its sensitivity to the short-range order structural characteristics of the absorbing atom. Also, being an element selective technique, XANES is complementary to techniques such as Raman spectroscopy, which provides information about vibrational modes associated with distinct polyatomic units. Results of an investigation of these glasses by Raman spectroscopy are given in the 1998 Annual Report. In Figure 3.5-5, the Al K-edge spectra are shown. For the glass synthesized at ambient pressure,
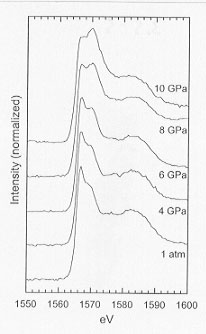 |
Fig. 3.5-5: XANES Al K-edge spectra of calcium aluminosilicate glasses formed up to 10 GPa. |
the spectrum is characterized by a sharp maximum at 1567 eV with a shoulder near 1570 eV and a broad, weaker absorption at about 1583 eV. These are all features consistent with Al in tetrahedral coordination when compared to standards of crystalline compounds such as albite and anorthite. For the glasses formed at pressures up to 6 GPa, we do not observe any significant changes in the Al K-edge apart from a very slight increase in the intensity of the shoulder near 1570 eV. However, for the glasses formed at pressures above 6 GPa, the intensity of the 1570 eV absorption increases dramatically, such that for the 10 GPa glass, it is the dominant feature in the spectrum. In contrast to the Al K-edge data, we observe no significant changes in either the Si K-edge or Ca K-edge spectra of these glasses over the entire range of pressure in which they were formed. These results suggest that the mechanisms involved in the compression of this aluminosilicate liquid are concentrated at the local environment surrounding Al3+ cations, consistent with previous studies of other aluminosilicate liquids using solid state NMR spectroscopy.
The rapid rise in the intensity of the absorption band at 1570 eV of the Al K-edge is likely to be correlated with an increase in the abundance of higher coordinated Al3+ cations, but may also be due to bond angle narrowing with an associated Al-O bond lengthening as demonstrated by recent results of multiple scattering calculations. Both of the above mechanisms should reduce the volume of the liquid at high pressures. In combination with the results of our earlier study of these glasses by Raman spectroscopy, we suggest that densification of the liquid upon initial pressurization to 6 GPa is more likely to involve an increase in oxygen coordination, a conclusion based on our Raman observations. However, at higher pressures, increasing cation coordination and bond angle narrowing become more favorable compression mechanisms.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page