

Knowledge of the rheological properties of silicate and aluminosilicate melts is of fundamental importance to understand and interpret correctly the physics of volcanic processes as well as the petrogenesis of magmatic rocks. The importance of an accurate and complete set of viscosity measurements derives from the extremely complex dependence of viscosity on composition, temperature and pressure. An important parameter to be considered when dealing with rheological properties of magma is the volatile content of the melt.
The low temperature viscosities of dry and hydrous XAlSi3O8 (X=Li, Na, K, Ca0.5, Mg0.5) melts have been investigated. The samples were hydrated via piston cylinder synthesis and the water contents subsequently determined by Karl Fischer titration (KFT) and IR spectroscopy. Both the anhydrous and hydrous viscosities were measured using the micropenetration technique in the range of viscosities between 108.5 to 1011.9 Pa s, at 1 atm pressure and in the temperature ranges of 745-990°C and 400-790°C for the dry and wet melts, respectively. The range of water content varied for all of the samples from 0.70 to 3.13 wt% H2O. The viscosities of dry melts vary, at fixed temperature, as a complex function of the identity of the cation in the order Li<Na<Ca Mg<K. This trend is interpreted as due to the combined effects of cation field strength and (Si, Al) distribution in these melts.
Mg<K. This trend is interpreted as due to the combined effects of cation field strength and (Si, Al) distribution in these melts.
With the introduction of water into these melts, the viscosity decreases for all of the compositions investigated. As water is further dissolved, the array of viscosities converges into two distinct curves, for alkali-bearing and alkaline-earth-bearing aluminosilicate liquids respectively (Fig. 3.5-7). In contrast to the insensitivity of viscosity to alkali cation identity for hydrous melts,
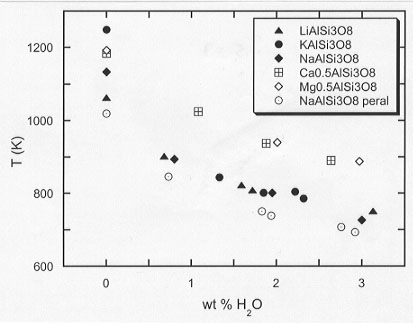 |
Fig. 3.5-7: Temperature required for a viscosity of 1010.5 Pa s as a function of water content. a LiAlSi3O8, b NaAlSi3O8, c KalSi3O8, d Na(1+x)AlSi3O(8+x) (Naexc), e Ca0.5AlSi3O8, f Mg0.5AlSi3O8. The data are presented for a viscosity of 1010.5 Pa s to avoid extrapolative comparison. |
the alkali/aluminium ratio remains an important parameter as we observe that the viscosity of a slightly peralkaline albite glass (Naexc) is lower than all of the others, both for the dry and the hydrous systems. We suggest that, in the case of alkaline-earth-bearing melts, an aluminium pair must be closely related to a doubly-charged cation, to maintain electrostatic neutrality. The increase in the size of smallest rearranging species that participates in the viscous flow process, as well as clustering of silica-rich and alumina-rich domains on a "intermediate-range" scale, may be the factors that result in the higher viscosities for Ca- and Mg-bearing compared to alkali-bearing liquids.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page