

Data on the rate at which minerals dissolve in silicate melts are important for our understanding of the residence time of xenoliths in magmas and the rate and chemical consequences of assimilation of foreign material by magmas. Unfortunately, the mineral dissolution rate database is very limited and interpretation and application of the data are complicated by the fact that the experiments have been carried out using a range of different experimental geometries, some of which are conducive to dissolution by a steady state process involving diffusion and convection, while the geometry used in other studies allows only diffusive dissolution.
The aim of the present study is to quantify the differences in dissolution rate and cation diffusion in experiments of different geometry. These experiments use quartz as the solute and basanite melt as the solvent and have been carried out at 0.5 GPa and 1350°C in the piston cylinder apparatus. To date the dissolution rate of quartz has been tested using four different sample geometries (Fig. 3.6-2).
There are considerable differences in the observed dissolution rate depending on the geometry of the sample (Fig. 3.6-2). In experiments where the quartz crystal is placed at the top of the capsule, it dissolves at a rate proportional to the square root of time, indicating that the rate controlling factor is diffusion alone. In experiments where the quartz crystal is placed at the bottom of the capsule the dissolution rate is proportional to time indicating that diffusion is no longer the rate limiting factor. Experiments in which the crystal is placed in the middle of the capsule and experiments with a quartz sphere at the top of the capsule give rates that are intermediate between the two extremes.
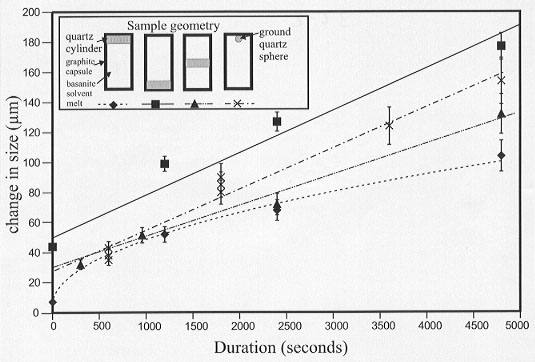 |
Fig. 3.6-2: Plot of dissolution amount vs. time for the four different sample geometries used (inset). Dissolution is proportional to t in the quartz-on-top experiments indicating that the rate is controlled by diffusion alone. In the other experiments dissolution is proportional to time indicating that diffusion is not the controlling process. |
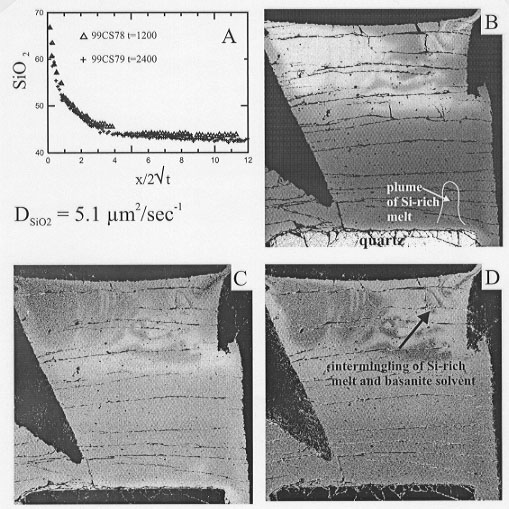 |
Fig. 3.6-3: A) Compositional profiles in 2 quartz-on-top experiments with concentration in wt% plotted against distance x (µm) divided by 2 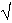 t(sec). Both profiles overlap indicating that dissolution is controlled by diffusion alone.
t(sec). Both profiles overlap indicating that dissolution is controlled by diffusion alone. |
B-D) X-ray maps of Si ka , K ka and Na ka respectively for a quartz-on-bottom experiment (t=2400 seconds). Regions of high concentration are light and regions of low concentration are dark. A thin contaminated layer showing local plume development marks the region adjacent to the quartz crystal. The top of the sample capsule shows complex intermingling of basanite (dark) and silica-rich melt that originally formed at the quartz - melt interface but was gravitationally unstable and rose through the denser basanite.
To understand the reason for the differences in the dissolution rates, detailed electron microprobe analyses were carried out on selected samples of each geometry. Samples with the quartz crystal at the top of the capsule give normal diffusion profiles in which the width of the contaminated boundary layer around the crystal increases as a function of the square root of time (Fig. 3.6-3a) and the dissolution is by diffusion only. All of the other experiments show anomalous profiles in which the boundary layer thickness is variable and the solvent melt has been extensively contaminated (Fig. 3.6-3b-d). The dissolution in these experiments proceeds initially via diffusion until the boundary layer around the crystal becomes gravitationally unstable and detaches from the dissolving crystal and rises into the capsule (a process known as compositional convection). This results in faster dissolution since the boundary layer thickness is considerably reduced. However, in experiments of longer duration the dissolution rate will decrease as the limited reservoir of solvent melt is contaminated. In nature, however, since the magma volume is much larger, dissolution by this process will always be faster than that occurring by diffusion alone. Therefore, measurement of both diffusive and convective-diffusive dissolution rates is important.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page