

Pegmatites are amongst the most complex geological systems of the Earth's crust and as such have been studied extensively. The most widely used model for their formation is that of Jahns and Burnham, which emphasises the role of water. However, it has now been demonstrated that co-existing fluids and melts will form, at certain pressures and temperatures, a single 'supercritical phase' that is neither fluid nor melt. A critical curve can even be drawn in P,T space, above which the system will be supercritical. As it has been shown in the literature that the presence of components common in pegmatitic systems (e.g. B or F) can affect the behaviour of granitic melts, this study has examined the effect of such components on the critical curve.
A series of albite glasses were synthesised containing either 5 or 10 wt% of excess Na, B, or F and 3.5 wt% water at 1.5 kbars and 1100°C for seven days in rapid quench TZM autoclaves. Once synthesised, small pieces of glass, water and an air bubble were loaded into a hydrothermal diamond anvil cell, which allows for the direct observation of complete miscibility between the melt and the aqueous fluid.
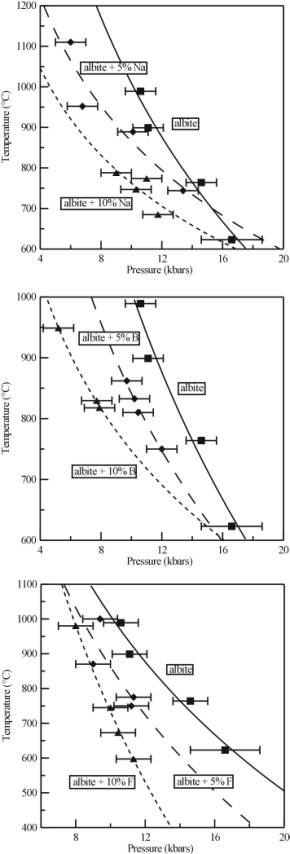
The results of the experiments are shown in Figure 3.4-7. As can be seen, all of the components added to the pure albite - H2O system affect the critical curve. For albite plus excess Na, the critical curve is lowered in temperature (by 143°C and 247°C at 10 kbars for 5 and 10 wt% Na respectively), and the gradient of the curve has changed. In a similar fashion, the presence of F and B also lowers the critical curve in temperature (by 147°C and 276°C at 10 kbars for 5 and 10 wt% F respectively; 168°C and 262°C at 10 kbars for albite + 5 and 10 wt% B respectively).
When combined with the results from an earlier reconnaissance study (see 1999 Annual Report), it is clear that the effects of the single additional components are roughly additive, and that the presence of more than one will significantly increase the magnitude of the effect. Therefore, in the complex chemical systems that are characteristic of pegmatites, complete miscibility between melt and fluid at moderate temperatures and pressures could be important. Any supercritical phase will have characteristics unlike those of either the fluid or the melt. Around the critical point, small changes in temperature and pressure will produce large density fluctuations in the system, and these will dramatically effect the transport properties of the melt, and could provide a mechanism for the growth of large crystals commonly found in pegmatites. There is also the possibility of interaction between the supercritical fluid and the surrounding country rock. The solvent properties of such a supercritical fluid are likely to be complex and may be affected by small fluctuations in temperature and pressure in an analogous fashion to supercritical CO2, which is used as an industrial solvent. Finally, once the conditions have dropped below the critical curve, the supercritical phase will unmix and during this process chemical components will partition between the fluid and the melt. The partitioning behaviour may be very different from that normally seen in geological systems.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page