

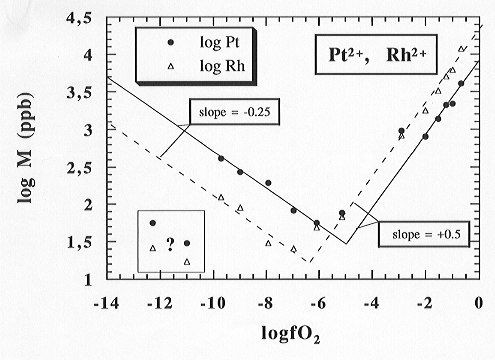
The experiments concerning the solubilities of Pt and Rh in a haplobasaltic melt were extended to a broader range of fO2's: low oxygen fugacities were achieved using CO/CO2 gas mixtures while high oxygen fugacities were achieved using O2-Ar gas mixtures (1, 3, 6, 10%, and finally pure O2). In a final step the fO2 was reversed to a low value of log fO2 = -11.96 using forming gas bubbled through an ice-water mixture. ICP-MS techniques were used for the determination of the Pt and Rh contents of the samples. The results shown in Fig. 3.2-3 confirm previous results about the Pt and Rh solubilities: both show principally the same solubility behaviour as observed by Ir, which appears to be typical for highly siderophile elements. Starting at high oxygen fugacity, a decrease of fO2 results in a decrease of the solubility of both elements. After a minimum solubility of Pt around log fO2 = -6 of around 60 ppb and 25 ppb for Rh, a further decrease of oxygen fugacity results in a sudden increase in solubility up to 160 ppb for Rh and 500 ppb for Pt at an oxygen fugacity of log fO2 = -10. Two data points at lower oxygen fugacities result from experimental problems and cannot yet be explained completely.
The data indicate a formal oxidation state of Pt and Rh of 2+ at high
oxygen fugacity. Results from experiments at an oxygen fugacity around
10-12 controlled by forming gas - ice water mixtures
(a C-free system) will be measured soon and might provide information on
the influence of C on the solubility of highly siderophile elements.
 |
|
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page