

The lower mantle, which constitutes more than half of the Earth's interior by volume, is believed to consist predominantly of (Mg,Fe)SiO3 perovskite with up to approximately 20% (Mg,Fe)O.
In the lower mantle system FeO-MgO-SiO2, the majority
of iron occurs as Fe2+, and partitions preferentially
into (Mg,Fe)O relative to the perovskite phase. Recent experiments by Wood
and Rubie (Science, 273: 1522-24, 1996), however, have shown that Fe-Mg
partitioning is drastically altered by the addition of 4-5 wt% Al2O3
to the system, resulting in an essentially equal partitioning of iron between
the perovskite and (Mg,Fe)O phases. One explanation for this behaviour
is a significant change in Fe3+/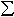 Fe of the perovskite phase.
Fe of the perovskite phase.
Multianvil experiments on quenched samples of (Mg,Fe)SiO3
perovskite synthesised at varying oxygen fugacities at 26 GPa and 1600°C
show that Fe3+/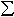 Fe in
the perovskite phase can reach approximately 20% under oxidising conditions.
The addition of a small amount of Al2O3
to the system, however, dramatically increases the Fe3+
content: with addition of 3 wt% Al2O3
Fe3+/
Fe in
the perovskite phase can reach approximately 20% under oxidising conditions.
The addition of a small amount of Al2O3
to the system, however, dramatically increases the Fe3+
content: with addition of 3 wt% Al2O3
Fe3+/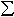 Fe values of more
than 50% in the perovskite phase were obtained. Figure 3.2-7
Fe values of more
than 50% in the perovskite phase were obtained. Figure 3.2-7
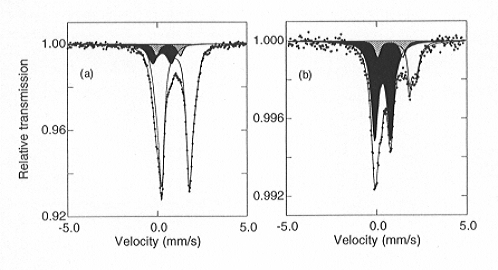 |
Fig. 3.2-7: Mössbauer spectra of (Mg,Fe)SiO3 perovskite: (a) Al-free; (b) containing 3.3 mol% Al2O3. The quadrupole doublets are shaded as follows: Fe2+ - no shading; Fe3+ - black; Fe2.5+ - grey. Note the dramatic increase in relative Fe3+ content of the perovskite phase in the presence of Al. |
illustrates Mössbauer spectra showing the dramatic increase in Fe3+ content of (Mg,Fe)SiO3 perovskite when Al2O3 is added. The relative amount of Fe3+ is strongly correlated to the Al content, and the substitution Mg2+ + Si4+ -> Fe3+ + Al3+ appears to be instrumental in stabilising Fe3+. Based on the estimated Al content of the lower mantle, these results suggest that the majority of iron in the perovskite phase is Fe3+, which has significant implications for the physical and chemical properties of the lower mantle.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page