

Halogens are minor elements in a wide range of magmatic compositions
found in both intrusive complexes and volcanic centres. Although both Cl
and F are considered volatile, Cl possesses a far higher volatility in
most naturally occurring melts than F. This difference in their volatilities
could have significant implications for magmatic and volcanic systems where
the halogen concentration of either can exceed 1 wt%. Fluorine concentrations
are high in certain rhyolitic volcanics where evidence of dry, but relatively
fluid effusive activity exists and the experimental evidence for a fluxing
effect of fluorine is abundant. Chlorine concentrations are highest in
more alkaline and less silicic systems such as pantellerites, yet there
are extremely few reliable data for the influence of Cl on melt viscosity.
Encouraged by evidence of pyromagmatic rocks very rich in Cl and Fe as
well as the high Fe contents of pantellerites in comparison with other
peralkaline natural melts, syntheses of melts in the Na-Si-O-Cl system
have been attempted. Successful syntheses of melts with up to 2 wt% Cl
have been achieved. Such melts have been subjected to viscometry using
the concentric cylinder and the micropenetration methods. The addition
of a few wt% Cl to melts in this system results in a slight decrease in
viscosity (Fig. 3.6-3). This influence of chlorine on viscosity appears
to contrast strongly with the influence of fluorine. Further investigations
are underway to systematize the influence of Cl on the viscosity of simple
melt compositions as well as to evaluate the effect in natural Fe-Cl rich
compositions such as pantellerites.
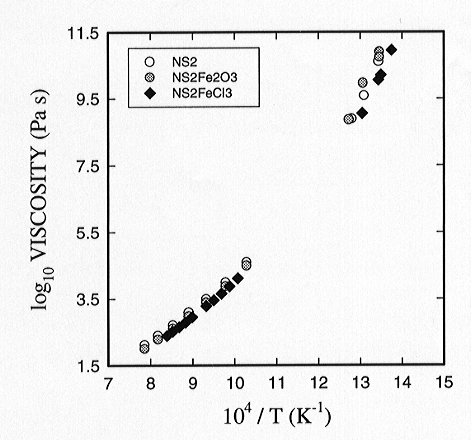 |
Fig 3.6-3: Log viscosity as a function of reciprocal temperature. The addition of Cl reduces the viscosity slightly at low temperatures. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page