

The abundances of high field strength elements have been used to investigate a variety of processes, such as partial melting, fractional crystallization and fluid metasomatism. Some HFSE pairs, most notably Nb-Ta and Zr-Hf, are so-called geochemical twins because both elements have virtually the same ionic radius and charge, resulting in the occurrence of many similar chemical and physical properties. However there are differences, and understanding why in some cases the behaviour of geochemical twins is nearly identical and in other cases they diverge is fundamental to the interpretation of trace element geochemical data.
Relative changes of activity coefficients of these elements in melts can be determined from differences in the solubilities of the end-members of solid solutions, specifically manganocolumbite-manganotantalite (MnNb2O6-MnTa2O6) and zircon-hafnon (ZrSiO4-HfSiO4). The solubility of columbite-tantalite at 800°C, 2 kbar and H2O-saturated conditions increases by about two orders of magnitude (Fig. 3.6-6a) from subaluminous (ASI=1) to peralkaline (ASI=0.5) melts due to changes of activity coefficients of Nb2O5 and Ta2O5 in the melt [ASI=Al/(Na+K+2Mn)]. Solubility products (Ksp) are related to the thermodynamic equilibrium constants (K) through the equations
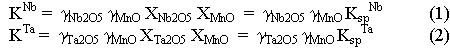
where 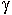 are activity coefficients and X are
molar fractions in the melt phase. If one divides equation 1 by 2, the
activity coefficient for MnO cancels out and one obtains the relationship:
are activity coefficients and X are
molar fractions in the melt phase. If one divides equation 1 by 2, the
activity coefficient for MnO cancels out and one obtains the relationship:
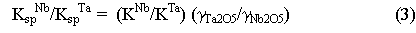
Therefore, at constant pressure and temperature, the ratio of the solubility
products of columbite and tantalite gives a number directly proportional
to the ratio of the activity coefficients of Ta2O5
and Nb2O5 in the melt. Over a change in melt composition
from ASI=0.5 to 1.0, 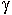 Ta2O5/
Ta2O5/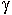 Nb2O5 apparently decreases by more than a factor of two (Fig.
3.6-6b).
Nb2O5 apparently decreases by more than a factor of two (Fig.
3.6-6b).
 |
Fig. 3.6-6: Solubility products (Ksp) of columbite-tantalite. a) in peralkaline to subaluminous granitic melts at 800°C, 2 kbar and water saturated conditions. b) Solubility product ratio KspNb/KspTa, which is proportional to the activity coefficient ratio |
The relative equilibrium fractionation of Nb and Ta between any phase and a silicate melt will thus depend strongly on melt composition. An example is rutile, which to a large extent controls the behaviour of Nb and Ta during the anatexis of metamorphic rocks as well as during the final stages of magmatic crystallization. The partitioning of Nb and Ta between melt and rutile can be described by equilibria of the type
This exchange reaction can be modelled using the columbite and tantalite solubility data if the solid solution between rutile and the columbite or tantalite component is ideal at magmatic temperatures and the solubility of the iron end-members Fe(Nb,Ta)2O6 is similar to those of the manganese end-members Mn(Nb,Ta)2O6. The calculated partition coefficients of Nb and Ta between rutile and peralkaline melts are similar to experimental data for trachytes and andesites (~30 to 50), but increase by about two orders of magnitude in subaluminous or peraluminous granitic magmas (~2000 to 7000). Any attempt to directly determine these partition coefficients experimentally would probably fail due to the sluggish reaction kinetics in granitic systems.
The ratio DNb/DTa is independent of FeO and MnO and from the estimated partition coefficients a systematic increase of this ratio from andesites to trachytes to peralkaline and peraluminous granites is apparent. This is due to changes in activity coefficients in the melt, implying that melt composition will affect the relative partition coefficients DNb/DTa of any phase. DNb/DTa should increase by a factor of four to five when going from andesitic to subaluminous or peraluminous granitic melts. This has fundamental consequences for the relative fractionation of Nb and Ta in the Earth´s crust.
Figure 3.6-7 seems to indicate that there is no systematic variation
of Nb/Ta with degree of fractionation for several alkaline and calc-alkaline
lava suites, ranging from basaltic to rhyolitic compositions. By contrast,
Nb/Ta decreases systematically with increasing fractionation of peraluminous
granites and rhyolites. In basaltic to andesitic melts, the solubility
of accessory phases concentrating high-field strength elements (e.g. rutile)
is high.
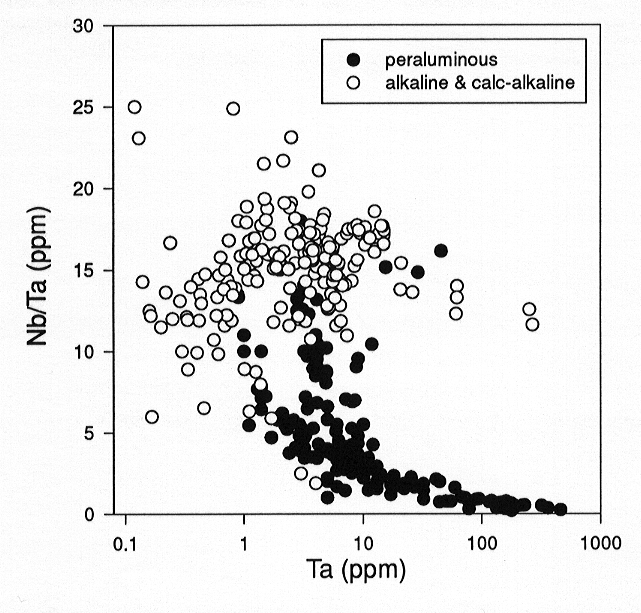 |
Fig. 3.6-7: Variation of the Nb/Ta ratio with Ta in natural alkaline and calc-alkaline volcanic rocks (white circles) and peraluminous granites and rhyolites (black circles). |
Therefore, such phases will either be absent during fractional crystallisation or will only appear in minor quantities. Moreover, these phases fractionate Nb and Ta only slightly and the partition coefficients are relatively low. In subaluminous or peraluminous granites, however, the solubility of accessory phases such as rutile is very low, they significantly fractionate Nb from Ta, and partition coefficients are extremely high. Therefore, even small amounts of accessory phases in peraluminous granitic systems will cause a strong decrease of Nb/Ta in residual liquids. Accordingly, there is no need to invoke fractionation by a fluid phase to explain the low Nb/Ta ratio characteristic for parts of the continental crust.
The success of the modelling of Nb-Ta has prompted a systematic investigation of Zr-Hf using zircon-hafnon. Obtaining relatively coarse-grained (>1 µm) hafnon was initially a problem, but this has been solved by synthesizing hafnon from a stoichiometric oxide mix with 4% HF at 1400°C and 15 kbar. A Zr-Hf solubility study analogous to columbite-tantalite is now being conducted.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page