

Determining the solubility of noble gases in melts at high pressures is important to our understanding of the degassing of Earth´s mantle. Noble gas transport is thought to be controlled by partial melting and the dissolution of noble gases in the melt. Thus, modelling the degassing process requires data on noble gas solubility in silicate melts and minerals.
The solubility of Ar and Xe in synthetic haplogranitic and tholeiitic melts was determined experimentally in the pressure range of 1 to 11 GPa and temperatures between 1500 and 2000°C. Experiments were performed in a piston-cylinder apparatus (1 to 3 GPa, 3/4 and ½ inch assemblies) and a 1200 t multi-anvil apparatus (2 to 10 GPa, 25/17 and 18/11 assemblies). Sample capsules contained 2 to 10 mg glass powder and the noble gas of interest. Noble gases were loaded into the capsules using a gas loading apparatus and were sealed by welding. Between 40 and 100 bars of the gas were loaded in this way, resulting in 5 to 15 wt% of the charge, sufficient to saturate the sample. For piston-cylinder and some multi-anvil experiments (25/17 assemblies) the sample capsule was inserted into a mould of pressed MgO and then enclosed in a second capsule, which then was also sealed by welding. Experiments were held for 30 to 60 minutes at the desired P and T and were quenched by shutting off the furnace power. The melts quenched to clear glasses containing few large bubbles and in few cases some crystals.
The noble gas concentration in the glasses was determined using the electron microprobe. For this purpose a standard with known noble gas concentration was available for Ar but not for Xe. This problem was overcome by preparing Xe-saturated albite glasses at 1.5 and 2.0 GPa and 1500° C, for which the Xe solubility had been previously reported. As a consequence the error for the absolute concentration may be larger for Xe than for Ar, but it has no consequence for the relative evolution of the Xe solubility as a function of pressure.
 |
 |
 of the microprobe analysis of at least
15 points measured for each glass. In some cases the error is smaller than
the symbol size of the microprobe analysis of at least
15 points measured for each glass. In some cases the error is smaller than
the symbol size |
|
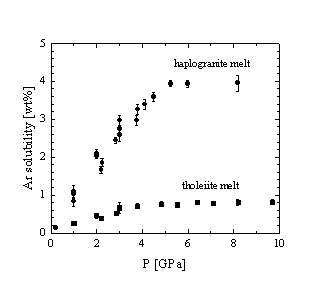
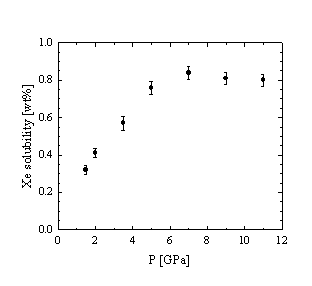
As a function of pressure the Ar solubility increases linearly up to about 4 - 5 GPa where it reaches 0.75 and 3.9 wt% for the tholeiitic and haplogranitic melt, respectively (Fig. 3.5-6a). At higher P only a small increase in the amount of dissolved Ar is observed, suggesting that some threshold concentration is reached. This would imply that the interstitial sites in the melt structure, suitable for the accommodation of Ar atoms are fully occupied. A very similar evolution with pressure is observed for the Xe solubility in tholeiitic melt (Fig. 3.5-6b).
Our results are different from those of Chamorro-Perez et al. (Earth Planet. Sci. Lett., 145, p 97, 1996; Nature 393, p 352, 1998)who reported a significant drop of Ar solubility in silica and San Carlos olivine melt at around 5 GPa, suggesting that melt densification results in an abrupt decrease of the hole size distribution for accommodating noble gas atoms. Our data show that such an effect is absent for our samples at least up to 11 GPa. A geochemical consequence of our results is that noble gases remain incompatible elements at pressure conditions covering most of the upper mantle. Mantle reservoirs with primordial noble gas signatures have therefore never experienced melt extraction.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page