

Boron, which is a minor component in granitic magmas and associated fluids, has considerable effects on melting temperature, viscosity and water solubility of granitic melts. Although the incorporation mechanisms of B in technical borate and borosilicate glasses have been studied to a great extent, we have little information about the structural role of B in melts and glasses of geological interest. In particular, the microscopic interaction of water with B-bearing glasses is nearly unknown. The aim of this study is to provide such information using a combined IR, Raman and NMR spectroscopic study.
Albite glasses containing different amounts of B2O3 (4.8, 9.1, 16.7 wt% B2O3) were synthesised at 1 atm and 1600° C. Hydrous glasses containing 4.4 ± 0.1 wt% water were prepared from the dry glasses and distilled water at 2 kbar and 1000° C in TZM rapid quench autoclaves. All glasses were analysed with IR micro-spectroscopy (Bruker IFS120HR), Raman micro-spectroscopy (Dilor XY) and NMR spectroscopy (Bruker MSL 200 at BGI, Chemagnetics Infinity 360 and 600 at Warwick, UK).
The results show that B is incorporated in the glass structure dominantly as trigonal BO3 groups, although some tetrahedral BO4 is also present (Fig. 3.5-7). There is a very good agreement between Raman and 11B NMR spectroscopic results, demonstrating that the amount of BO3 in the glasses increases linearly with increasing total boron content. The hydrous glasses contain more BO4 groups compared to the dry glasses, suggesting that this species may be stabilised by water or by the higher pressure at which the hydrous samples were prepared. The changes in the Raman and NMR (29Si, 27Al) spectra with increasing B-content show that B interacts with the aluminosilicate network, probably by formation of Si-O-B and possibly Al-O-B units. For the hydrous glasses significant changes in the NIR spectra were observed with increasing B-content, indicating important changes in the water speciation. With increasing B-concentration the intensity of the 5200 cm-1 band decreases, reflecting a decrease of the molecular H2O concentration. The band at 4500 cm-1, related to structurally bonded OH-groups, gains in intensity and additional features develop at about 4150 and 4640 cm-1. The observed changes in the NIR spectra are consistent with those previously reported in the literature for B-containing haplogranite glasses, and are probably due to the formation of B-OH complexes. However, the exact nature of such complexes cannot be deduced from the present data.
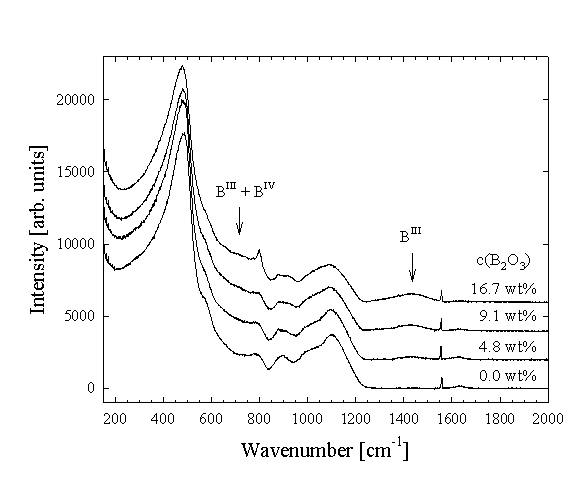 |
The water speciation (as molecular H2O and total amount of structurally bonded OH-groups) was determined quantitatively with low temperature 1H NMR spectroscopy. The results show a linear increase of the hydroxyl concentration at the expense of molecular H2O with increasing B-content (Fig. 3.5-8). For example, the H2O concentration in the glass with 16.7 wt% added B2O3 is reduced to about 50% of that in the B-free glass.
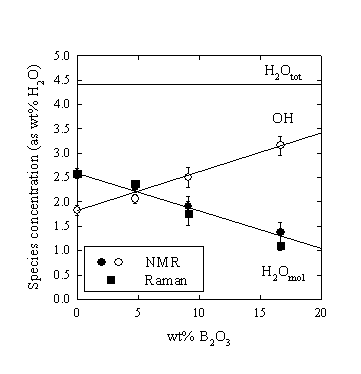 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page